Temporal dynamics of a homeostatic pathway controlling neural network activity
- PMID: 24065881
- PMCID: PMC3776619
- DOI: 10.3389/fnmol.2013.00028
Temporal dynamics of a homeostatic pathway controlling neural network activity
Abstract
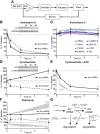
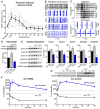
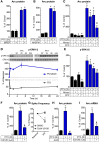
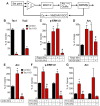
Neurons use a variety of mechanisms to homeostatically regulate neural network activity in order to maintain firing in a bounded range. One such process involves the bi-directional modulation of excitatory synaptic drive in response to chronic changes in network activity. Down-scaling of excitatory synapses in response to high activity requires Arc-dependent endocytosis of glutamate receptors. However, the temporal dynamics and signaling pathways regulating Arc during homeostatic plasticity are not well understood. Here we determine the relative contribution of transcriptional and translational control in the regulation of Arc, the signaling pathways responsible for the activity-dependent production of Arc, and the time course of these signaling events as they relate to the homeostatic adjustment of network activity in hippocampal neurons. We find that an ERK1/2-dependent transcriptional pathway active within 1-2 h of up-regulated network activity induces Arc leading to a restoration of network spiking rates within 12 h. Under basal and low activity conditions, specialized mechanisms are in place to rapidly degrade Arc mRNA and protein such that they have half-lives of less than 1 h. In addition, we find that while mTOR signaling is regulated by network activity on a similar time scale, mTOR-dependent translational control is not a major regulator of Arc production or degradation suggesting that the signaling pathways underlying homeostatic plasticity are distinct from those mediating synapse-specific forms of synaptic depression.
Keywords: Arc; ERK signaling; TSC/mTOR; activity-dependent gene regulation; hippocampus; homeostatic plasticity; multi-electrode array; network activity.
Figures
References
-
- Antion M. D., Hou L., Wong H., Hoeffer C. A., Klann E. (2008). mGluR-dependent long-term depression is associated with increased phosphorylation of S6 and synthesis of elongation factor 1A but remains expressed in S6K-deficient mice. Mol. Cell. Biol. 28, 2996–3007 10.1128/MCB.00201-08 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

