A thermosensitive mutation alters the effects of lacosamide on slow inactivation in neuronal voltage-gated sodium channels, NaV1.2
- PMID: 24065921
- PMCID: PMC3778253
- DOI: 10.3389/fphar.2013.00121
A thermosensitive mutation alters the effects of lacosamide on slow inactivation in neuronal voltage-gated sodium channels, NaV1.2
Abstract
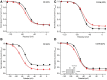
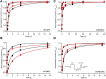
Epilepsy is a disorder characterized by seizures and convulsions. The basis of epilepsy is an increase in neuronal excitability that, in some cases, may be caused by functional defects in neuronal voltage gated sodium channels (NaVs). The C121W mutation of the β1 subunit, in particular, gives rise to the thermosensitive generalized epilepsy with febrile seizures plus (GEFS+) phenotype. Lacosamide is used to treat epileptic seizures and is distinct from other anti-seizure drugs by targeting NaV slow-inactivation. We studied the effects of a physiologically relevant concentration of lacosamide on the biophysical properties of NaV1.2 channels associated with either WT-β1 or the mutant C121W-β1 subunit. Biophysical parameters were measured at both normal (22°C) and elevated (34°C) temperatures to elicit the differential temperature-sensitivity of C121W. Lacosamide was more effective in NaV1.2 associated with the WT-β1 than with C121W-β1 at either temperature. There is also a more potent effect by lacosamide on slow inactivation at elevated temperatures. Our data suggest a modulatory role is imparted by the β1 subunit in the interaction between the drug and the channel.
Keywords: C121W-β1; GEFS+; WT-β1 (wild-type); lacosamide; slow inactivation; thermosensitive; voltage-gated sodium channels.
Figures
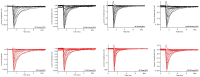
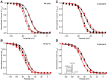
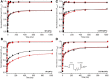
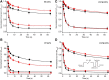
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

