Regulated mucin secretion from airway epithelial cells
- PMID: 24065956
- PMCID: PMC3776272
- DOI: 10.3389/fendo.2013.00129
Regulated mucin secretion from airway epithelial cells
Abstract
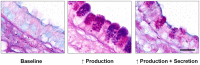
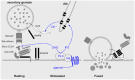
Secretory epithelial cells of the proximal airways synthesize and secrete gel-forming polymeric mucins. The secreted mucins adsorb water to form mucus that is propelled by neighboring ciliated cells, providing a mobile barrier which removes inhaled particles and pathogens from the lungs. Several features of the intracellular trafficking of mucins make the airway secretory cell an interesting comparator for the cell biology of regulated exocytosis. Polymeric mucins are exceedingly large molecules (up to 3 × 10(6) Da per monomer) whose folding and initial polymerization in the ER requires the protein disulfide isomerase Agr2. In the Golgi, mucins further polymerize to form chains and possibly branched networks comprising more than 20 monomers. The large size of mucin polymers imposes constraints on their packaging into transport vesicles along the secretory pathway. Sugar side chains account for >70% of the mass of mucins, and their attachment to the protein core by O-glycosylation occurs in the Golgi. Mature polymeric mucins are stored in large secretory granules ∼1 μm in diameter. These are translocated to the apical membrane to be positioned for exocytosis by cooperative interactions among myristoylated alanine-rich C kinase substrate, cysteine string protein, heat shock protein 70, and the cytoskeleton. Mucin granules undergo exocytic fusion with the plasma membrane at a low basal rate and a high stimulated rate. Both rates are mediated by a regulated exocytic mechanism as indicated by phenotypes in both basal and stimulated secretion in mice lacking Munc13-2, a sensor of the second messengers calcium and diacylglycerol (DAG). Basal secretion is induced by low levels of activation of P2Y2 purinergic and A3 adenosine receptors by extracellular ATP released in paracrine fashion and its metabolite adenosine. Stimulated secretion is induced by high levels of the same ligands, and possibly by inflammatory mediators as well. Activated receptors are coupled to phospholipase C by Gq, resulting in the generation of DAG and of IP3 that releases calcium from apical ER. Stimulated secretion requires activation of the low affinity calcium sensor Synaptotagmin-2, while a corresponding high affinity calcium sensor in basal secretion is not known. The core exocytic machinery is comprised of the SNARE proteins VAMP8, SNAP23, and an unknown Syntaxin protein, together with the scaffolding protein Munc18b. Common and distinct features of this exocytic system in comparison to neuroendocrine cells and neurons are highlighted.
Keywords: MARCKS; Munc13; Munc18; exocytosis; mucin; mucus; secretion; synaptotagmin.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

