Patterns of rotavirus vaccine uptake and use in privately-insured US infants, 2006-2010
- PMID: 24066076
- PMCID: PMC3774785
- DOI: 10.1371/journal.pone.0073825
Patterns of rotavirus vaccine uptake and use in privately-insured US infants, 2006-2010
Abstract
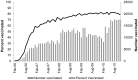
Rotavirus vaccines are highly effective at preventing gastroenteritis in young children and are now universally recommended for infants in the US. We studied patterns of use of rotavirus vaccines among US infants with commercial insurance. We identified a large cohort of infants in the MarketScan Research Databases, 2006-2010. The analysis was restricted to infants residing in states without state-funded rotavirus vaccination programs. We computed summary statistics and used multivariable regression to assess the association between patient-, provider-, and ecologic-level variables of rotavirus vaccine receipt and series completion. Approximately 69% of 594,117 eligible infants received at least one dose of rotavirus vaccine from 2006-2010. Most infants received the rotavirus vaccines at the recommended ages, but more infants completed the series for monovalent rotavirus vaccine than pentavalent rotavirus vaccine or a mix of the vaccines (87% versus 79% versus 73%, P<0.001). In multivariable analyses, the strongest predictors of rotavirus vaccine series initiation and completion were receipt of the diphtheria, tetanus and acellular pertussis vaccine (Initiation: RR = 7.91, 95% CI = 7.69-8.13; Completion: RR = 1.26, 95% CI = 1.23-1.29), visiting a pediatrician versus family physician (Initiation: RR = 1.51, 95% CI = 1.49-1.52; Completion: RR = 1.13, 95% CI = 1.11-1.14), and living in a large metropolitan versus smaller metropolitan, urban, or rural area. We observed rapid diffusion of the rotavirus vaccine in routine practice; however, approximately one-fifth of infants did not receive at least one dose of vaccine as recently as 2010. Interventions to increase rotavirus vaccine coverage should consider targeting family physicians and encouraging completion of the vaccine series.
Conflict of interest statement
Figures

References
-
- Dennehy PH (2005) Acute diarrheal disease in children: Epidemiology, prevention, and treatment. Infect Dis Clin North Am 19: 585–602 doi:10.1016/j.idc.2005.05.003 - DOI - PubMed
-
- Cortes JE, Curns AT, Tate JE, Cortese MM, Patel MM, et al. (2011) Rotavirus vaccine and health care utilization for diarrhea in U.S. children. N Engl J Med 365: 1108–1117 doi:10.1056/NEJMoa1000446 - DOI - PubMed
-
- Parashar UD, Alexander JP, Glass RI, Advisory Committee on Immunization Practices (ACIP), Centers for Disease Control and Prevention (CDC) (2006) Prevention of rotavirus gastroenteritis among infants and children. recommendations of the advisory committee on immunization practices (ACIP). MMWR Recomm Rep 55: 1–13. - PubMed
-
- Cortese MM, Parashar UD, Centers for Disease Control and Prevention (CDC) (2009) Prevention of rotavirus gastroenteritis among infants and children: Recommendations of the advisory committee on immunization practices (ACIP). MMWR Recomm Rep 58: 1–25. - PubMed
-
- Centers for Disease Control and Prevention (CDC) (2012) National, state, and local area vaccination coverage among children aged 19–35 months – United States, 2011. MMWR Morb Mortal Wkly Rep 61: 689–696. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

