Single nucleotide polymorphism array profiling of adrenocortical tumors--evidence for an adenoma carcinoma sequence?
- PMID: 24066089
- PMCID: PMC3774745
- DOI: 10.1371/journal.pone.0073959
Single nucleotide polymorphism array profiling of adrenocortical tumors--evidence for an adenoma carcinoma sequence?
Abstract
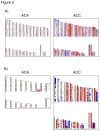
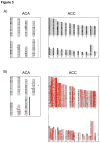
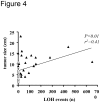
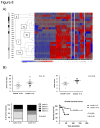
Adrenocortical tumors consist of benign adenomas and highly malignant carcinomas with a still incompletely understood pathogenesis. A total of 46 adrenocortical tumors (24 adenomas and 22 carcinomas) were investigated aiming to identify novel genes involved in adrenocortical tumorigenesis. High-resolution single nucleotide polymorphism arrays (Affymetrix) were used to detect copy number alterations (CNAs) and copy neutral losses of heterozygosity (cnLOH). Genomic clustering showed good separation between adenomas and carcinomas, with best partition including only chromosome 5, which was highly amplified in 17/22 malignant tumors. The malignant tumors had more relevant genomic aberrations than benign tumors, such as a higher median number of recurrent CNA (2631 vs 94), CNAs >100 Kb (62.5 vs 7) and CN losses (72.5 vs 5.5), and a higher percentage of samples with cnLOH (91% vs 29%). Within the carcinoma cohort, a precise genetic pattern (i.e. large gains at chr 5, 7, 12, and 19, and losses at chr 1, 2, 13, 17, and 22) was associated with a better prognosis (overall survival: 72.2 vs 35.4 months, P=0.063). Interestingly, >70% of gains frequent in benign were also present in malignant tumors. Notch signaling was the most frequently involved pathway in both tumor entities. Finally, a CN gain at imprinted "IGF2" locus chr 11p15.5 appeared to be an early alteration in a multi-step tumor progression, followed by the loss of one or two alleles, associated with increased IGF2 expression, only in carcinomas. Our study serves as database for the identification of genes and pathways, such as Notch signaling, which could be involved in the pathogenesis of adrenocortical tumors. Using these data, we postulate an adenoma-carcinoma sequence for these tumors.
Conflict of interest statement
Figures




Similar articles
-
Single nucleotide polymorphism microarray analysis in cortisol-secreting adrenocortical adenomas identifies new candidate genes and pathways.Neoplasia. 2012 Mar;14(3):206-18. doi: 10.1593/neo.111758. Neoplasia. 2012. PMID: 22496620 Free PMC article.
-
Clinical and pathophysiological implications of chromosomal alterations in adrenocortical tumors: an integrated genomic approach.J Clin Endocrinol Metab. 2012 Feb;97(2):E301-11. doi: 10.1210/jc.2011-1588. Epub 2011 Nov 23. J Clin Endocrinol Metab. 2012. PMID: 22112813
-
Analysis of genomic alterations in sporadic adrenocortical lesions. Gain of chromosome 17 is an early event in adrenocortical tumorigenesis.Am J Pathol. 1999 Oct;155(4):1039-45. doi: 10.1016/S0002-9440(10)65205-4. Am J Pathol. 1999. PMID: 10514385 Free PMC article.
-
[Sporadic adrenocortical tumors: genetics and perspectives for the pathologist].Ann Pathol. 2008 Oct;28(5):409-16. doi: 10.1016/j.annpat.2008.07.005. Epub 2008 Oct 9. Ann Pathol. 2008. PMID: 19068395 Review. French.
-
Pediatric adrenocortical tumors: molecular events leading to insulin-like growth factor II gene overexpression.J Clin Endocrinol Metab. 2000 May;85(5):2048-56. doi: 10.1210/jcem.85.5.6589. J Clin Endocrinol Metab. 2000. PMID: 10843195 Review.
Cited by
-
Identifying New Potential Biomarkers in Adrenocortical Tumors Based on mRNA Expression Data Using Machine Learning.Cancers (Basel). 2021 Sep 17;13(18):4671. doi: 10.3390/cancers13184671. Cancers (Basel). 2021. PMID: 34572898 Free PMC article.
-
Transformation of a Benign Adrenocortical Adenoma to a Metastatic Adrenocortical Carcinoma Is Rare But It Happens.JCEM Case Rep. 2024 Jul 30;2(8):luae131. doi: 10.1210/jcemcr/luae131. eCollection 2024 Aug. JCEM Case Rep. 2024. PMID: 39081696 Free PMC article.
-
Recurrent activating mutation in PRKACA in cortisol-producing adrenal tumors.Nat Genet. 2014 Jun;46(6):613-7. doi: 10.1038/ng.2956. Epub 2014 Apr 20. Nat Genet. 2014. PMID: 24747643 Free PMC article.
-
Therapeutic Targets for Adrenocortical Carcinoma in the Genomics Era.J Endocr Soc. 2018 Sep 26;2(11):1259-1274. doi: 10.1210/js.2018-00197. eCollection 2018 Nov 1. J Endocr Soc. 2018. PMID: 30402590 Free PMC article. Review.
-
Adrenal cancer in 2013: Time to individualize treatment for adrenocortical cancer?Nat Rev Endocrinol. 2014 Feb;10(2):76-8. doi: 10.1038/nrendo.2013.263. Epub 2014 Jan 7. Nat Rev Endocrinol. 2014. PMID: 24393782 Review.
References
-
- Bilimoria KY, Shen WT, Elaraj D, Bentrem DJ, Winchester DJ et al. (2008) Adrenocortical carcinoma in the United States: treatment utilization and prognostic factors. Cancer 113: 3130-3136. doi:10.1002/cncr.23886. PubMed: 18973179. - DOI - PubMed
-
- Allolio B, Fassnacht M (2006) Clinical review: Adrenocortical carcinoma: clinical update. J Clin Endocrinol Metab 91: 2027-2037. doi:10.1210/jc.2005-2639. PubMed: 16551738. - DOI - PubMed
-
- Libè R, Fratticci A, Bertherat J (2007) Adrenocortical cancer: pathophysiology and clinical management. Endocr Relat Cancer 14: 13-28. doi:10.1677/erc.1.01130. PubMed: 17395972. - DOI - PubMed
-
- Fassnacht M, Libé R, Kroiss M, Allolio B (2011) Adrenocortical carcinoma: a clinician’s update. Nat Rev Endocrinol 7: 323-335. doi:10.1038/nrendo.2010.235. PubMed: 21386792. - DOI - PubMed
-
- Icard P, Goudet P, Charpenay C, Andreassian B, Carnaille B et al. (2001) Adrenocortical carcinomas: surgical trends and results of a 253-patient series from the French Association of Endocrine Surgeons study group. World J Surg 25: 891-897. doi:10.1007/s00268-001-0047-y. PubMed: 11572030. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

