Assessing tumor contrast in radiographically dense breast tissue using Diffuse Optical Spectroscopic Imaging (DOSI)
- PMID: 24066941
- PMCID: PMC3979060
- DOI: 10.1186/bcr3485
Assessing tumor contrast in radiographically dense breast tissue using Diffuse Optical Spectroscopic Imaging (DOSI)
Abstract
Introduction: Radiographic density adversely affects the performance of X-ray mammography and can be particularly problematic in younger and high-risk women. Because of this limitation, there is significant ongoing effort to develop alternative cancer screening and detection strategies for this population. This pilot study evaluates the potential of Diffuse Optical Spectroscopic Imaging (DOSI) to image known tumors in dense breast tissue.
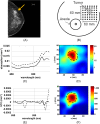
Methods: We performed a retrospective analysis on 24 radiographically dense breast cancer subjects measured with DOSI over a four-year period (Breast Imaging Reporting and Data System - BI-RADS, category 3 and 4, average age = 39 ± 7.6, average maximum size 31 ± 1 7 mm). Two previously-described DOSI contrast functions, the tissue optical index (TOI) and the specific tumor component (STC), which are based upon the concentrations and spectral signatures of hemoglobin, water and lipids, respectively, were used to form 2D optical images of breast tumors.
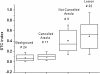
Results: Using TOI and STC, 21 out of 24 breast tumors were found to be statistically different from the surrounding highly vascularized dense tissue and to be distinguishable from the areolar region. For these patients, the tumor to normal contrast was 2.6 ± 1.2 (range 1.3 to 5.5) and 10.0 ± 7.5 (range 3.3 to 26.4) for TOI and STC, respectively. STC images were particularly useful in eliminating metabolic background from the retroareolar region which led to identification of two out of four retroareolar tumors.
Conclusions: Using both the abundance and the disposition of the tissue chromophores recovered from the DOSI measurements, we were able to observe tumor contrast relative to dense breast tissue. These preliminary results suggest that DOSI spectral characterization strategies may provide new information content that could help imaging breast tumors in radiographically dense tissue and in particular in the areolar complex.
Figures


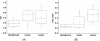
References
-
- Hersh MR. Imaging the dense breast. Appl Radiol. 2004;15:22.
-
- Bevers TB, Anderson BO, Bonaccio E, Buys S, Daly MB, Dempsey PJ, Farrar WB, Fleming I, Garber JE, Harris RE, Helvie M, Hoover S, Krontiras H, Shaw S, Singletary E, Sugg Skinner C, Smith ML, Tsangaris TN, Wiley EL, Williams C. National Comprehensive Cancer Network. Breast cancer screening and diagnosis. J Natl Compr Canc Netw. 2009;15:1060–1096. - PubMed
-
- Berg WA, Blume JD, Cormack JB, Mendelson EB, Lehrer D, Böhm-Vélez M, Pisano ED, Jong RA, Evans WP, Morton MJ, Mahoney MC, Larsen LH, Barr RG, Farria DM, Marques HS, Boparai K. ACRIN 6666 Investigators. Combined screening with ultrasound and mammography vs mammography alone in women at elevated risk of breast cancer. JAMA. 2008;15:2151–2163. doi: 10.1001/jama.299.18.2151. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

