Physical characterization of the manganese-sensing pneumococcal surface antigen repressor from Streptococcus pneumoniae
- PMID: 24067066
- PMCID: PMC3859839
- DOI: 10.1021/bi401132w
Physical characterization of the manganese-sensing pneumococcal surface antigen repressor from Streptococcus pneumoniae
Abstract
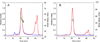
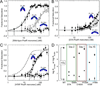
Transition metals, including manganese, are required for the proper virulence and persistence of many pathogenic bacteria. In Streptococcus pneumoniae (Spn), manganese homeostasis is controlled by a high-affinity Mn(II) uptake complex, PsaBCA, and a constitutively expressed efflux transporter, MntE. psaBCA expression is transcriptionally regulated by the DtxR/MntR family metalloregulatory protein pneumococcal surface antigen repressor (PsaR) in Spn. Here, we present a comprehensive analysis of the metal and DNA binding properties of PsaR. PsaR is a homodimer in the absence and presence of metals and binds two manganese or zinc atoms per protomer (four per dimer) in two pairs of structurally distinct sites, termed site 1 and site 2. Site 1 is likely filled with Zn(II) in vivo (K(Zn1) ≥ 10¹³ M⁻¹; K(Mn1) ≈ 10⁸ M⁻¹). The Zn(II)-site 1 complex adopts a pentacoordinate geometry as determined by X-ray absorption spectroscopy containing a single cysteine and appears to be analogous to the Cd(II) site observed in Streptococcus gordonii ScaR. Site 1 is necessary but not sufficient for full positive allosteric activation of DNA operator binding by metals as measured by ΔGc, the allosteric coupling free energy, because site 1 mutants show an intermediate ΔGc. Site 2 is the primary regulatory site and governs specificity for Mn(II) over Zn(II) in PsaR, where ΔGc(Zn,Mn) >> ΔGc(Zn,Zn) despite the fact that Zn(II) binds site 2 with an affinity 40-fold higher than that of Mn(II); i.e., K(Zn2) > K(Mn2). Mutational studies reveal that Asp7 in site 2 is a critical ligand for Mn(II)-dependent allosteric activation of DNA binding. These findings are discussed in the context of other well-studied DtxR/MntR Mn(II)/Fe(II) metallorepressors.
Figures


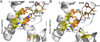

References
-
- Andreini C, Bertini I, Cavallaro G, Holliday GL, Thornton JM. Metal ions in biological catalysis: from enzyme databases to general principles. J Biol Inorg Chem. 2008;13:1205–1218. - PubMed
-
- Waldron KJ, Rutherford JC, Ford D, Robinson NJ. Metalloproteins and metal sensing. Nature. 2009;460:823–830. - PubMed
-
- Klein JS, Lewinson O. Bacterial ATP-driven transporters of transition metals: physiological roles, mechanisms of action, and roles in bacterial virulence. Metallomics. 2011;3:1098–1108. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

