Dysregulation of the complement cascade in the hSOD1G93A transgenic mouse model of amyotrophic lateral sclerosis
- PMID: 24067070
- PMCID: PMC3850877
- DOI: 10.1186/1742-2094-10-119
Dysregulation of the complement cascade in the hSOD1G93A transgenic mouse model of amyotrophic lateral sclerosis
Abstract
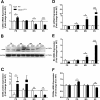
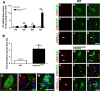
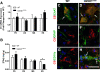
Background: Components of the innate immune complement system have been implicated in the pathogenesis of amyotrophic lateral sclerosis (ALS); however, a comprehensive examination of complement expression in this disease has not been performed. This study therefore aimed to determine the expression of complement components (C1qB, C4, factor B, C3/C3b, C5 and CD88) and regulators (CD55 and CD59a) in the lumbar spinal cord of hSOD1(G93A) mice during defined disease stages.
Methods: hSOD1(G93A) and wild-type mice were examined at four different ages of disease progression. mRNA and protein expression of complement components and regulators were examined using quantitative PCR, western blotting and ELISA. Localisation of complement components within lumbar spinal cord was investigated using immunohistochemistry. Statistical differences between hSOD1(G93A) and wild-type mice were analysed using a two-tailed t-test at each stage of disease progression.
Results: We found several early complement factors increased as disease progressed, whilst complement regulators decreased; suggesting overall increased complement activation through the classical or alternative pathways in hSOD1(G93A) mice. CD88 was also increased during disease progression, with immunolocalisation demonstrating expression on motor neurons and increasing expression on microglia surrounding the regions of motor neuron death.
Conclusions: These results indicate that local complement activation and increased expression of CD88 may contribute to motor neuron death and ALS pathology in the hSOD1(G93A) mouse. Hence, reducing complement-induced inflammation could be an important therapeutic strategy to treat ALS.
Figures


References
-
- Woodruff TM, Costantini KJ, Taylor SM, Noakes PG. Role of complement in motor neuron disease: animal models and therapeutic potential of complement inhibitors. Adv Exp Med Biol. 2008;632:143–158. - PubMed
-
- Lee JD, Lee JY, Taylor SM, Noakes PG, Woodruff TM. In: Amyotrophic Lateral Sclerosis. Maurer MH, editor. Croatia: InTech; 2012. Innate Immunity in ALS; pp. 393–412.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

