Dominant optic atrophy, OPA1, and mitochondrial quality control: understanding mitochondrial network dynamics
- PMID: 24067127
- PMCID: PMC3856479
- DOI: 10.1186/1750-1326-8-32
Dominant optic atrophy, OPA1, and mitochondrial quality control: understanding mitochondrial network dynamics
Abstract
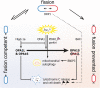
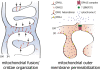
Mitochondrial quality control is fundamental to all neurodegenerative diseases, including the most prominent ones, Alzheimer's Disease and Parkinsonism. It is accomplished by mitochondrial network dynamics - continuous fission and fusion of mitochondria. Mitochondrial fission is facilitated by DRP1, while MFN1 and MFN2 on the mitochondrial outer membrane and OPA1 on the mitochondrial inner membrane are essential for mitochondrial fusion. Mitochondrial network dynamics are regulated in highly sophisticated ways by various different posttranslational modifications, such as phosphorylation, ubiquitination, and proteolytic processing of their key-proteins. By this, mitochondria process a wide range of different intracellular and extracellular parameters in order to adapt mitochondrial function to actual energetic and metabolic demands of the host cell, attenuate mitochondrial damage, recycle dysfunctional mitochondria via the mitochondrial autophagy pathway, or arrange for the recycling of the complete host cell by apoptosis. Most of the genes coding for proteins involved in this process have been associated with neurodegenerative diseases. Mutations in one of these genes are associated with a neurodegenerative disease that originally was described to affect retinal ganglion cells only. Since more and more evidence shows that other cell types are affected as well, we would like to discuss the pathology of dominant optic atrophy, which is caused by heterozygous sequence variants in OPA1, in the light of the current view on OPA1 protein function in mitochondrial quality control, in particular on its function in mitochondrial fusion and cytochrome C release. We think OPA1 is a good example to understand the molecular basis for mitochondrial network dynamics.
Figures

References
-
- Grandemange S, Herzig S, Martinou JC. Mitochondrial dynamics and cancer. Semin Cancer Biol. 2009;19(1):50–56. - PubMed
-
- Wang W, Lu J, Zhu F, Wei J, Jia C, Zhang Y, Zhou L, Xie H, Zheng S. Pro-apoptotic and anti-proliferative effects of mitofusin-2 via Bax signaling in hepatocellular carcinoma cells. Med Oncol. 2012;29(1):70–76. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

