Interferon-induced transmembrane protein 3 is a type II transmembrane protein
- PMID: 24067232
- PMCID: PMC3820858
- DOI: 10.1074/jbc.M113.514356
Interferon-induced transmembrane protein 3 is a type II transmembrane protein
Abstract
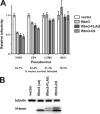
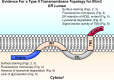
The interferon-induced transmembrane (IFITM) proteins are a family of small membrane proteins that inhibit the cellular entry of several genera of viruses. These proteins had been predicted to adopt a two-pass, type III transmembrane topology with an intracellular loop, two transmembrane helices (TM1 and TM2), and extracellular N and C termini. Recent work, however, supports an intramembrane topology for the helices with cytosolic orientation of both termini. Here we determined the topology of murine Ifitm3. We found that the N terminus of Ifitm3 could be stained by antibodies at the cell surface but that this conformation was cell type-dependent and represented a minority of the total plasma membrane pool. In contrast, the C terminus was readily accessible to antibodies at the cell surface and extracellular C termini comprised most or all of those present at the plasma membrane. The addition of a C-terminal KDEL endoplasmic reticulum retention motif to Ifitm3 resulted in sequestration of Ifitm3 in the ER, demonstrating an ER-luminal orientation of the C terminus. C-terminal, but not N-terminal, epitope tags were also degraded within lysosomes, consistent with their luminal orientation. Furthermore, epitope-tagged Ifitm3 TM2 functioned as a signal anchor sequence when expressed in isolation. Collectively, our results demonstrate a type II transmembrane topology for Ifitm3 and will provide insight into its interaction with potential targets and cofactors.
Keywords: Flow Cytometry; Ifitm3; Interferon; Membrane Proteins; Microscopic Imaging; Virus Entry.
Figures

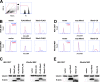
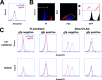
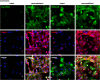
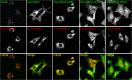



References
-
- Huang I.-C., Bailey C. C., Weyer J. L., Radoshitzky S. R., Becker M. M., Chiang J. J., Brass A. L., Ahmed A. A., Chi X., Dong L., Longobardi L. E., Boltz D., Kuhn J. H., Elledge S. J., Bavari S., Denison M. R., Choe H., Farzan M. (2011) Distinct patterns of IFITM-mediated restriction of filoviruses, SARS coronavirus, and influenza A virus. PLoS Pathog. 7, e1001258. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

