Increased extracellular glutamate in the nucleus accumbens promotes excessive ethanol drinking in ethanol dependent mice
- PMID: 24067300
- PMCID: PMC3895249
- DOI: 10.1038/npp.2013.256
Increased extracellular glutamate in the nucleus accumbens promotes excessive ethanol drinking in ethanol dependent mice
Abstract
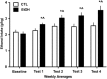
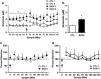
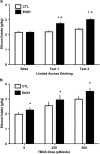
Using a well-established model of ethanol dependence and relapse, this study examined adaptations in glutamatergic transmission in the nucleus accumbens (NAc) and their role in regulating voluntary ethanol drinking. Mice were first trained to drink ethanol in a free-choice, limited access (2 h/day) paradigm. One group (EtOH mice) received repeated weekly cycles of chronic intermittent ethanol (CIE) exposure with intervening weeks of test drinking sessions, whereas the remaining mice (CTL mice) were similarly treated but did not receive CIE treatment. Over repeated cycles of CIE exposure, EtOH mice exhibited significant escalation in drinking (up to ∼3.5 g/kg), whereas drinking remained relatively stable at baseline levels (2-2.5 g/kg) in CTL mice. Using in vivo microdialysis procedures, extracellular glutamate (GLUEX) levels in the NAc were increased approximately twofold in EtOH mice compared with CTL mice, and this difference was observed 7 days after final CIE exposure, indicating that this hyperglutamatergic state persisted beyond acute withdrawal. This finding prompted additional studies examining the effects of pharmacologically manipulating GLUEX in the NAc on ethanol drinking in the CIE model. The non-selective glutamate reuptake antagonist, threo-β-benzyloxyaspartate (TBOA), was bilaterally microinjected into the NAc and found to dose-dependently increase drinking in nondependent (CTL) mice to levels attained by dependent (EtOH) mice. TBOA also further increased drinking in EtOH mice. In contrast, reducing glutamatergic transmission in the NAc via bilateral injections of the metabotropic glutamate receptor-2/3 agonist LY379268 reduced drinking in dependent (EtOH) mice to nondependent (CTL) levels, whereas having a more modest effect in decreasing ethanol consumption in CTL mice. Taken together, these data support an important role of glutamatergic transmission in the NAc in regulating ethanol drinking. Additionally, these results indicate that ethanol dependence produces adaptations that favor elevated glutamate activity in the NAc which, in turn, promote excessive levels of ethanol consumption associated with dependence.
Figures


References
-
- Amara SG, Fontana ACK. Excitatory amino acid transporters: keeping up with glutamate. Neurochem Int. 2002;41:313–318. - PubMed
-
- Becker HC, Lopez MF. Increased ethanol drinking after repeated chronic ethanol exposure and withdrawal experience in C57BL/6 mice. Alcohol Clin Exp Res. 2004;28:1829–1838. - PubMed
-
- Berger L, Fisher M, Brondino M, Bohn M, Gwyther R, Longo L, et al. Efficacy of acamprosate for alcohol dependence in a family medicine setting in the United States: a randomized, double-blind, placebo-controlled study. Alcohol Clin Exp Res. 2013;37:668–674. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

