Adaptor complex AP2/PICALM, through interaction with LC3, targets Alzheimer's APP-CTF for terminal degradation via autophagy
- PMID: 24067654
- PMCID: PMC3801056
- DOI: 10.1073/pnas.1315110110
Adaptor complex AP2/PICALM, through interaction with LC3, targets Alzheimer's APP-CTF for terminal degradation via autophagy
Abstract
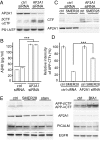
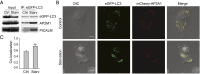
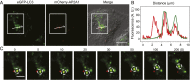
The hallmarks of Alzheimer's disease (AD) are the aggregates of amyloid-β (Aβ) peptides and tau protein. Autophagy is a major cellular pathway leading to the removal of aggregated proteins. We have reported recently that autophagy was responsible for amyloid precursor protein cleaved C-terminal fragment (APP-CTF) degradation and amyloid β clearance in an Atg5-dependent manner. Here we aimed to elucidate the molecular mechanism by which autophagy mediates the degradation of APP-CTF and the clearance of amyloid β. Through affinity purification followed by mass spectrum analysis, we identified adaptor protein (AP) 2 together with phosphatidylinositol clathrin assembly lymphoid-myeloid leukemia (PICALM) as binding proteins of microtubule-associated protein 1 light chain 3 (LC3). Further analysis showed that AP2 regulated the cellular levels of APP-CTF. Knockdown of AP2 reduced autophagy-mediated APP-CTF degradation. Immunoprecipitation and live imaging analysis demonstrated that AP2 and PICALM cross-link LC3 with APP-CTF. These data suggest that the AP-2/PICALM complex functions as an autophagic cargo receptor for the recognition and shipment of APP-CTF from the endocytic pathway to the LC3-marked autophagic degradation pathway. This molecular mechanism linking AP2/PICALM and AD is consistent with genetic evidence indicating a role for PICALM as a risk factor for AD.
Keywords: aggregate removal; endocytosis; trafficking.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Perez RG, et al. Mutagenesis identifies new signals for beta-amyloid precursor protein endocytosis, turnover, and the generation of secreted fragments, including Abeta42. J Biol Chem. 1999;274(27):18851–18856. - PubMed
-
- Koo EH, Squazzo SL, Selkoe DJ, Koo CH. Trafficking of cell-surface amyloid beta-protein precursor. I. Secretion, endocytosis and recycling as detected by labeled monoclonal antibody. J Cell Sci. 1996;109(Pt 5):991–998. - PubMed
-
- Canfield WM, Johnson KF, Ye RD, Gregory W, Kornfeld S. Localization of the signal for rapid internalization of the bovine cation-independent mannose 6-phosphate/insulin-like growth factor-II receptor to amino acids 24-29 of the cytoplasmic tail. J Biol Chem. 1991;266(9):5682–5688. - PubMed
-
- Jadot M, Canfield WM, Gregory W, Kornfeld S. Characterization of the signal for rapid internalization of the bovine mannose 6-phosphate/insulin-like growth factor-II receptor. J Biol Chem. 1992;267(16):11069–11077. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

