Properties of Slo1 K+ channels with and without the gating ring
- PMID: 24067659
- PMCID: PMC3799338
- DOI: 10.1073/pnas.1313433110
Properties of Slo1 K+ channels with and without the gating ring
Abstract
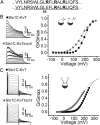
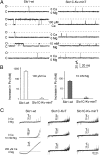
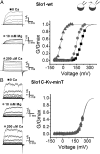
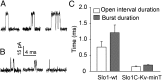
High-conductance Ca(2+)- and voltage-activated K(+) (Slo1 or BK) channels (KCNMA1) play key roles in many physiological processes. The structure of the Slo1 channel has two functional domains, a core consisting of four voltage sensors controlling an ion-conducting pore, and a larger tail that forms an intracellular gating ring thought to confer Ca(2+) and Mg(2+) sensitivity as well as sensitivity to a host of other intracellular factors. Although the modular structure of the Slo1 channel is known, the functional properties of the core and the allosteric interactions between core and tail are poorly understood because it has not been possible to study the core in the absence of the gating ring. To address these questions, we developed constructs that allow functional cores of Slo1 channels to be expressed by replacing the 827-amino acid gating ring with short tails of either 74 or 11 amino acids. Recorded currents from these constructs reveals that the gating ring is not required for either expression or gating of the core. Voltage activation is retained after the gating ring is replaced, but all Ca(2+)- and Mg(2+)-dependent gating is lost. Replacing the gating ring also right-shifts the conductance-voltage relation, decreases mean open-channel and burst duration by about sixfold, and reduces apparent mean single-channel conductance by about 30%. These results show that the gating ring is not required for voltage activation but is required for Ca(2+) and Mg(2+) activation. They also suggest possible actions of the unliganded (passive) gating ring or added short tails on the core.
Keywords: BK channel; Kv1.4; iberiotoxin; tetraethylammonium; β1 subunit.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Nelson MT, et al. Relaxation of arterial smooth muscle by calcium sparks. Science. 1995;270(5236):633–637. - PubMed
-
- Brenner R, et al. Vasoregulation by the beta1 subunit of the calcium-activated potassium channel. Nature. 2000;407(6806):870–876. - PubMed
-
- Robitaille R, Garcia ML, Kaczorowski GJ, Charlton MP. Functional colocalization of calcium and calcium-gated potassium channels in control of transmitter release. Neuron. 1993;11(4):645–655. - PubMed
-
- Fettiplace R, Fuchs PA. Mechanisms of hair cell tuning. Annu Rev Physiol. 1999;61:809–834. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

