Fast vesicle transport is required for the slow axonal transport of synapsin
- PMID: 24068803
- PMCID: PMC3782618
- DOI: 10.1523/JNEUROSCI.1148-13.2013
Fast vesicle transport is required for the slow axonal transport of synapsin
Erratum in
-
Erratum: Tang et al., "Fast Vesicle Transport Is Required for the Slow Axonal Transport of Synapsin".J Neurosci. 2019 Jul 10;39(28):5627. doi: 10.1523/JNEUROSCI.0976-19.2019. Epub 2019 May 28. J Neurosci. 2019. PMID: 31138659 Free PMC article. No abstract available.
Abstract
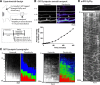
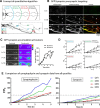
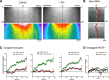
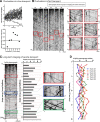
Although it is known that cytosolic/soluble proteins synthesized in cell bodies are transported at much lower overall velocities than vesicles in fast axonal transport, the fundamental basis for this slow movement is unknown. Recently, we found that cytosolic proteins in axons of mouse cultured neurons are conveyed in a manner that superficially resembles diffusion, but with a slow anterograde bias that is energy- and motor-dependent (Scott et al., 2011). Here we show that slow axonal transport of synapsin, a prototypical member of this rate class, is dependent upon fast vesicle transport. Despite the distinct overall dynamics of slow and fast transport, experimentally induced and intrinsic variations in vesicle transport have analogous effects on slow transport of synapsin as well. Dynamic cotransport of vesicles and synapsin particles is also seen in axons, consistent with a model where higher-order assemblies of synapsin are conveyed by transient and probabilistic associations with vesicles moving in fast axonal transport. We posit that such dynamic associations generate the slow overall anterogradely biased flow of the population ("dynamic-recruitment model"). Our studies uncover the underlying kinetic basis for a classic cytosolic/soluble protein moving in slow axonal transport and reveal previously unknown links between slow and fast transport, offering a clearer conceptual picture of this curious phenomenon.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
