Early BDNF treatment ameliorates cell loss in the entorhinal cortex of APP transgenic mice
- PMID: 24068826
- PMCID: PMC3782628
- DOI: 10.1523/JNEUROSCI.5195-12.2013
Early BDNF treatment ameliorates cell loss in the entorhinal cortex of APP transgenic mice
Abstract
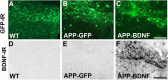
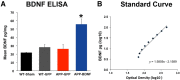
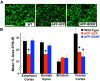
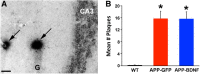
Brain-derived neurotrophic factor (BDNF) improves molecular, cellular, and behavioral measures of neural dysfunction in genetic models of Alzheimer's disease (Blurton-Jones et al., 2009; Nagahara et al., 2009). However, BDNF treatment after disease onset has not been reported to improve neuronal survival in these models. We now report prevention of neuronal loss with early life BDNF treatment in mutant mice expressing two amyloid precursor protein (APP) mutations associated with early-onset familial Alzheimer's disease. APP transgenic mice underwent lentiviral BDNF gene delivery into the entorhinal cortices at age 2 months and were examined 5 months later. BDNF-treated mice exhibited significant improvements in hippocampal-dependent contextual fear conditioning compared with control-treated APP mice (p < 0.05). Stereological analysis of entorhinal cortical cell number demonstrated ∼20% reductions in neuronal number in layers II-VI of the entorhinal cortex in untreated APP mutant mice compared with wild-type mice (p < 0.0001), and significant amelioration of cell loss by BDNF (p < 0.001). Moreover, BDNF gene delivery improved synaptophysin immunoreactivity in the entorhinal cortex and, through anterograde BDNF transport, in the hippocampus (p < 0.01). Notably, BDNF did not affect amyloid plaque numbers, indicating that direct amyloid reduction is not necessary to achieve significant neuroprotective benefits in mutant amyloid models of Alzheimer's disease.
Figures


References
-
- Blurton-Jones M, Kitazawa M, Martinez-Coria H, Castello NA, Müller FJ, Loring JF, Yamasaki TR, Poon WW, Green KN, LaFerla FM. Neural stem cells improve cognition via BDNF in a transgenic model of Alzheimer disease. Proc Natl Acad Sci U S A. 2009;106:13594–13599. doi: 10.1073/pnas.0901402106. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
