Sodium-mediated plateau potentials in lumbar motoneurons of neonatal rats
- PMID: 24068829
- PMCID: PMC6618457
- DOI: 10.1523/JNEUROSCI.1483-13.2013
Sodium-mediated plateau potentials in lumbar motoneurons of neonatal rats
Abstract
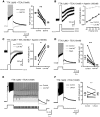
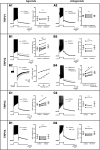
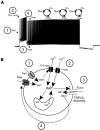
The development and the ionic nature of bistable behavior in lumbar motoneurons were investigated in rats. One week after birth, almost all (∼80%) ankle extensor motoneurons recorded in whole-cell configuration displayed self-sustained spiking in response to a brief depolarization that emerged when the temperature was raised >30°C. The effect of L-type Ca(2+) channel blockers on self-sustained spiking was variable, whereas blockade of the persistent sodium current (I(NaP)) abolished them. When hyperpolarized, bistable motoneurons displayed a characteristic slow afterdepolarization (sADP). The sADPs generated by repeated depolarizing pulses summed to promote a plateau potential. The sADP was tightly associated with the emergence of Ca(2+) spikes. Substitution of extracellular Na(+) or chelation of intracellular Ca(2+) abolished both sADP and the plateau potential without affecting Ca(2+) spikes. These data suggest a key role of a Ca(2+)-activated nonselective cation conductance ((CaN)) in generating the plateau potential. In line with this, the blockade of (CaN) by flufenamate abolished both sADP and plateau potentials. Furthermore, 2-aminoethoxydiphenyl borate (2-APB), a common activator of thermo-sensitive vanilloid transient receptor potential (TRPV) cation channels, promoted the sADP. Among TRPV channels, only the selective activation of TRPV2 channels by probenecid promoted the sADP to generate a plateau potential. To conclude, bistable behaviors are, to a large extent, determined by the interplay between three currents: L-type I(Ca), I(NaP), and a Na(+)-mediated I(CaN) flowing through putative TRPV2 channels.
Figures







References
-
- Angstadt JD, Choo JJ. Sodium-dependent plateau potentials in cultured Retzius cells of the medicinal leech. J Neurophysiol. 1996;76:1491–1502. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
