Nanoparticles for Improving Cancer Diagnosis
- PMID: 24068857
- PMCID: PMC3779646
- DOI: 10.1016/j.mser.2013.03.001
Nanoparticles for Improving Cancer Diagnosis
Abstract
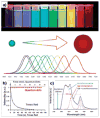
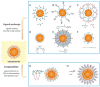
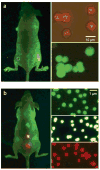
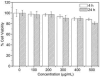
Despite the progress in developing new therapeutic modalities, cancer remains one of the leading diseases causing human mortality. This is mainly attributed to the inability to diagnose tumors in their early stage. By the time the tumor is confirmed, the cancer may have already metastasized, thereby making therapies challenging or even impossible. It is therefore crucial to develop new or to improve existing diagnostic tools to enable diagnosis of cancer in its early or even pre-syndrome stage. The emergence of nanotechnology has provided such a possibility. Unique physical and physiochemical properties allow nanoparticles to be utilized as tags with excellent sensitivity. When coupled with the appropriate targeting molecules, nanoparticle-based probes can interact with a biological system and sense biological changes on the molecular level with unprecedented accuracy. In the past several years, much progress has been made in applying nanotechnology to clinical imaging and diagnostics, and interdisciplinary efforts have made an impact on clinical cancer management. This article aims to review the progress in this exciting area with emphases on the preparation and engineering techniques that have been developed to assemble "smart" nanoprobes.
Keywords: bioconjugation; biomarkers; cancer diagnosis; imaging; nanomedicine; nanotechnology; surface modification.
Figures

















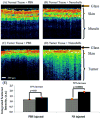


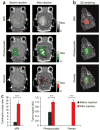
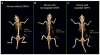
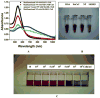
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
