Predicting odor perceptual similarity from odor structure
- PMID: 24068899
- PMCID: PMC3772038
- DOI: 10.1371/journal.pcbi.1003184
Predicting odor perceptual similarity from odor structure
Abstract
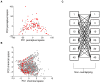
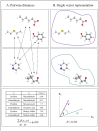
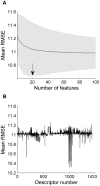
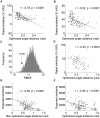
To understand the brain mechanisms of olfaction we must understand the rules that govern the link between odorant structure and odorant perception. Natural odors are in fact mixtures made of many molecules, and there is currently no method to look at the molecular structure of such odorant-mixtures and predict their smell. In three separate experiments, we asked 139 subjects to rate the pairwise perceptual similarity of 64 odorant-mixtures ranging in size from 4 to 43 mono-molecular components. We then tested alternative models to link odorant-mixture structure to odorant-mixture perceptual similarity. Whereas a model that considered each mono-molecular component of a mixture separately provided a poor prediction of mixture similarity, a model that represented the mixture as a single structural vector provided consistent correlations between predicted and actual perceptual similarity (r≥0.49, p<0.001). An optimized version of this model yielded a correlation of r = 0.85 (p<0.001) between predicted and actual mixture similarity. In other words, we developed an algorithm that can look at the molecular structure of two novel odorant-mixtures, and predict their ensuing perceptual similarity. That this goal was attained using a model that considers the mixtures as a single vector is consistent with a synthetic rather than analytical brain processing mechanism in olfaction.
Conflict of interest statement
The authors disclosed these findings to the Weizmann Institute Technology Licensing company (YEDA), and they have initiated application for patent on the described method and algorithm.
Figures


References
-
- Bell A (1914) Discovery and Invention. National Geographic 649.
-
- Wise P, Olsson M, Cain W (2000) Quantification of odor quality. Chem Senses 25: 429–443. - PubMed
-
- Kaeppler K, Mueller F (2013) Odor classification: a review of factors influencing perception-based odor arrangements. Chem Senses 38: 189–209. - PubMed
-
- Amoore JE (1970) The molecular basis of odor. Springfield: Charles C Thomas.
-
- Amoore JE (1977) Specific anosmia and the concept of primary odors. Chemical Senses and Flavor 2: 267–281.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

