Predictive modeling of in vivo response to gemcitabine in pancreatic cancer
- PMID: 24068909
- PMCID: PMC3777914
- DOI: 10.1371/journal.pcbi.1003231
Predictive modeling of in vivo response to gemcitabine in pancreatic cancer
Abstract
A clear contradiction exists between cytotoxic in-vitro studies demonstrating effectiveness of Gemcitabine to curtail pancreatic cancer and in-vivo studies failing to show Gemcitabine as an effective treatment. The outcome of chemotherapy in metastatic stages, where surgery is no longer viable, shows a 5-year survival <5%. It is apparent that in-vitro experiments, no matter how well designed, may fail to adequately represent the complex in-vivo microenvironmental and phenotypic characteristics of the cancer, including cell proliferation and apoptosis. We evaluate in-vitro cytotoxic data as an indicator of in-vivo treatment success using a mathematical model of tumor growth based on a dimensionless formulation describing tumor biology. Inputs to the model are obtained under optimal drug exposure conditions in-vitro. The model incorporates heterogeneous cell proliferation and death caused by spatial diffusion gradients of oxygen/nutrients due to inefficient vascularization and abundant stroma, and thus is able to simulate the effect of the microenvironment as a barrier to effective nutrient and drug delivery. Analysis of the mathematical model indicates the pancreatic tumors to be mostly resistant to Gemcitabine treatment in-vivo. The model results are confirmed with experiments in live mice, which indicate uninhibited tumor proliferation and metastasis with Gemcitabine treatment. By extracting mathematical model parameter values for proliferation and death from monolayer in-vitro cytotoxicity experiments with pancreatic cancer cells, and simulating the effects of spatial diffusion, we use the model to predict the drug response in-vivo, beyond what would have been expected from sole consideration of the cancer intrinsic resistance. We conclude that this integrated experimental/computational approach may enhance understanding of pancreatic cancer behavior and its response to various chemotherapies, and, further, that such an approach could predict resistance based on pharmacokinetic measurements with the goal to maximize effective treatment strategies.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
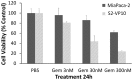
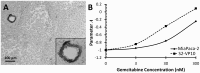
 (untreated) and
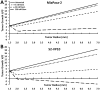
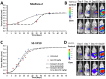
(untreated) and  (treated). (B) Bioluminescence signal shown for representative mice with S2-VP20 tumors. (C) S2-VP10 radii and fitting to Gompertz equations
(treated). (B) Bioluminescence signal shown for representative mice with S2-VP20 tumors. (C) S2-VP10 radii and fitting to Gompertz equations  (untreated) and
(untreated) and  (treated). (D) Bioluminescence signal shown for representative mice with S2-VP20 tumors. Error bars in (A) and (C) correspond to standard error of the mean.
(treated). (D) Bioluminescence signal shown for representative mice with S2-VP20 tumors. Error bars in (A) and (C) correspond to standard error of the mean.Similar articles
-
Myo-inositol trispyrophosphate-mediated hypoxia reversion controls pancreatic cancer in rodents and enhances gemcitabine efficacy.Int J Cancer. 2014 Jun 1;134(11):2572-82. doi: 10.1002/ijc.28597. Epub 2013 Nov 25. Int J Cancer. 2014. PMID: 24214898
-
Resistance of pancreatic cancer to gemcitabine treatment is dependent on mitochondria-mediated apoptosis.Int J Cancer. 2004 Mar 20;109(2):182-8. doi: 10.1002/ijc.11679. Int J Cancer. 2004. PMID: 14750167
-
Targeting cMET with INC280 impairs tumour growth and improves efficacy of gemcitabine in a pancreatic cancer model.BMC Cancer. 2015 Feb 19;15:71. doi: 10.1186/s12885-015-1064-9. BMC Cancer. 2015. PMID: 25884642 Free PMC article.
-
Treatment of metastatic pancreatic cancer.J Natl Compr Canc Netw. 2005 Sep;3(5):627-36. doi: 10.6004/jnccn.2005.0036. J Natl Compr Canc Netw. 2005. PMID: 16194454 Review.
-
Gemcitabine in the treatment of metastatic pancreatic cancer.Expert Rev Anticancer Ther. 2008 Apr;8(4):511-23. doi: 10.1586/14737140.8.4.511. Expert Rev Anticancer Ther. 2008. PMID: 18402518 Review.
Cited by
-
A tipping point in cancer-immune dynamics leads to divergent immunotherapy responses and hampers biomarker discovery.J Immunother Cancer. 2021 May;9(5):e002032. doi: 10.1136/jitc-2020-002032. J Immunother Cancer. 2021. PMID: 34059522 Free PMC article.
-
Simulation of the Protein-Shedding Kinetics of a Fully Vascularized Tumor.Cancer Inform. 2015 Dec 20;14:163-75. doi: 10.4137/CIN.S35374. eCollection 2015. Cancer Inform. 2015. PMID: 26715830 Free PMC article.
-
Modeling of Nanotherapy Response as a Function of the Tumor Microenvironment: Focus on Liver Metastasis.Front Bioeng Biotechnol. 2020 Aug 19;8:1011. doi: 10.3389/fbioe.2020.01011. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 32974325 Free PMC article. Review.
-
In Silico Oncology: Quantification of the In Vivo Antitumor Efficacy of Cisplatin-Based Doublet Therapy in Non-Small Cell Lung Cancer (NSCLC) through a Multiscale Mechanistic Model.PLoS Comput Biol. 2016 Sep 22;12(9):e1005093. doi: 10.1371/journal.pcbi.1005093. eCollection 2016 Sep. PLoS Comput Biol. 2016. PMID: 27657742 Free PMC article.
-
Pharmacokinetic/pharmacodynamic modeling of combination-chemotherapy for lung cancer.J Theor Biol. 2018 Jul 7;448:38-52. doi: 10.1016/j.jtbi.2018.03.035. Epub 2018 Apr 1. J Theor Biol. 2018. PMID: 29614265 Free PMC article.
References
-
- Burris HA 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, et al. (1997) Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 15: 2403–2413. - PubMed
-
- Berlin JD, Catalano P, Thomas JP, Kugler JW, Haller DG, et al. (2002) Phase III study of gemcitabine in combination with fluorouracil versus gemcitabine alone in patients with advanced pancreatic carcinoma: Eastern Cooperative Oncology Group Trial E2297. J Clin Oncol 20: 3270–3275. - PubMed
-
- Rocha Lima CM, Green MR, Rotche R, Miller WH Jr, Jeffrey GM, et al. (2004) Irinotecan plus gemcitabine results in no survival advantage compared with gemcitabine monotherapy in patients with locally advanced or metastatic pancreatic cancer despite increased tumor response rate. J Clin Oncol 22: 3776–3783. - PubMed
-
- Louvet C, Labianca R, Hammel P, Lledo G, Zampino MG, et al. (2005) Gemcitabine in combination with oxaliplatin compared with gemcitabine alone in locally advanced or metastatic pancreatic cancer: results of a GERCOR and GISCAD phase III trial. J Clin Oncol 23: 3509–3516. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

