Collective dynamics underlying allosteric transitions in hemoglobin
- PMID: 24068910
- PMCID: PMC3777908
- DOI: 10.1371/journal.pcbi.1003232
Collective dynamics underlying allosteric transitions in hemoglobin
Abstract
Hemoglobin is the prototypic allosteric protein. Still, its molecular allosteric mechanism is not fully understood. To elucidate the mechanism of cooperativity on an atomistic level, we developed a novel computational technique to analyse the coupling of tertiary and quaternary motions. From Molecular Dynamics simulations showing spontaneous quaternary transitions, we separated the transition trajectories into two orthogonal sets of motions: one consisting of intra-chain motions only (referred to as tertiary-only) and one consisting of global inter-chain motions only (referred to as quaternary-only). The two underlying subspaces are orthogonal by construction and their direct sum is the space of full motions. Using Functional Mode Analysis, we were able to identify a collective coordinate within the tertiary-only subspace that is correlated to the most dominant motion within the quaternary-only motions, hence providing direct insight into the allosteric coupling mechanism between tertiary and quaternary conformation changes. This coupling-motion is substantially different from tertiary structure changes between the crystallographic structures of the T- and R-state. We found that hemoglobin's allosteric mechanism of communication between subunits is equally based on hydrogen bonds and steric interactions. In addition, we were able to affect the T-to-R transition rates by choosing different histidine protonation states, thereby providing a possible atomistic explanation for the Bohr effect.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
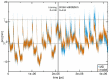
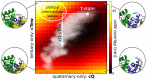
 -chain (green), the
-chain (green), the  -chain (blue) and the
-chain (blue) and the  -chain (yellow) of Hb is shown to illustrate the motions. Projections of the original simulation data onto this plane are shown as white dots.
-chain (yellow) of Hb is shown to illustrate the motions. Projections of the original simulation data onto this plane are shown as white dots.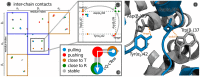
 and the
and the  residues in which one contact partner switches when going from T-state (orange) to the R-state (green). (C) Schematic representation of the contact classifications along cQ-cTew.
residues in which one contact partner switches when going from T-state (orange) to the R-state (green). (C) Schematic representation of the contact classifications along cQ-cTew.Similar articles
-
Crystallographic evidence for a new ensemble of ligand-induced allosteric transitions in hemoglobin: the T-to-T(high) quaternary transitions.Biochemistry. 2005 Apr 26;44(16):6101-21. doi: 10.1021/bi047813a. Biochemistry. 2005. PMID: 15835899
-
Spontaneous quaternary and tertiary T-R transitions of human hemoglobin in molecular dynamics simulation.PLoS Comput Biol. 2010 May 6;6(5):e1000774. doi: 10.1371/journal.pcbi.1000774. PLoS Comput Biol. 2010. PMID: 20463873 Free PMC article.
-
Coarse-grained and all-atom modeling of structural states and transitions in hemoglobin.Proteins. 2013 Feb;81(2):240-52. doi: 10.1002/prot.24180. Epub 2012 Oct 26. Proteins. 2013. PMID: 22987685
-
Evolution of allosteric models for hemoglobin.IUBMB Life. 2007 Aug-Sep;59(8-9):586-99. doi: 10.1080/15216540701272380. IUBMB Life. 2007. PMID: 17701554 Review.
-
Structures of a hemoglobin-based blood substitute: insights into the function of allosteric proteins.Structure. 1997 Feb 15;5(2):227-37. doi: 10.1016/s0969-2126(97)00181-0. Structure. 1997. PMID: 9032082 Review.
Cited by
-
Graph-theoretical analysis for energy landscape reveals the organization of state transitions in the resting-state human cerebral cortex.PLoS One. 2019 Sep 9;14(9):e0222161. doi: 10.1371/journal.pone.0222161. eCollection 2019. PLoS One. 2019. PMID: 31498822 Free PMC article.
-
Identifying coupled clusters of allostery participants through chemical shift perturbations.Proc Natl Acad Sci U S A. 2019 Feb 5;116(6):2078-2085. doi: 10.1073/pnas.1811168116. Epub 2019 Jan 24. Proc Natl Acad Sci U S A. 2019. PMID: 30679272 Free PMC article.
-
Exposing the distinctive modular behavior of β-strands and α-helices in folded proteins.Proc Natl Acad Sci U S A. 2020 Nov 17;117(46):28775-28783. doi: 10.1073/pnas.1920455117. Epub 2020 Nov 4. Proc Natl Acad Sci U S A. 2020. PMID: 33148805 Free PMC article.
-
Comment on 'Valid molecular dynamics simulations of human hemoglobin require a surprisingly large box size'.Elife. 2019 Jun 20;8:e44718. doi: 10.7554/eLife.44718. Elife. 2019. PMID: 31219782 Free PMC article.
-
Induced Fit in Protein Multimerization: The HFBI Case.PLoS Comput Biol. 2016 Nov 10;12(11):e1005202. doi: 10.1371/journal.pcbi.1005202. eCollection 2016 Nov. PLoS Comput Biol. 2016. PMID: 27832079 Free PMC article.
References
-
- Perutz MF, Wilkinson A, Paoli M, Dodson G (1998) The stereochemical mechanism of the cooperative effects in hemoglobin revisited. Annu Rev Biophys Biomol Struct 27: 1–34. - PubMed
-
- Eaton W, Henry E, Hofrichter J, Bettati S, Viappiani C, et al. (2007) Evolution of allosteric models for hemoglobin. IUBMB Life 59: 586–599. - PubMed
-
- Shadrina MS, English AM, Peslherbe GH (2012) Effective simulations of gas diffusion through kinetically accessible tunnels in multisubunit proteins: O2 pathways and escape routes in t-state deoxyhemoglobin. J Am Chem Soc 134: 11177–11184. - PubMed
-
- Lepeshkevich SV, Biziuk SA, Lemeza AM, Dzhagarov BM (2011) The kinetics of molecular oxygen migration in the isolated α chains of human hemoglobin as revealed by molecular dynamics simulations and laser kinetic spectroscopy. Biochim Biophys Acta 1814: 1279–1288. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

