Import of proteins into the peroxisomal matrix
- PMID: 24069002
- PMCID: PMC3781343
- DOI: 10.3389/fphys.2013.00261
Import of proteins into the peroxisomal matrix
Abstract
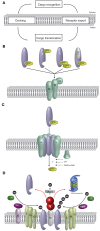
Peroxisomes constitute a dynamic compartment in all nucleated cells. They fulfill diverse metabolic tasks in response to environmental changes and cellular demands. This adaptation is implemented by modulation of the enzyme content of the organelles, which is accomplished by dynamically operating peroxisomal protein transport machineries. Soluble import receptors recognize their newly synthesized cargo proteins in the cytosol and ferry them to the peroxisomal membrane. Subsequently, the cargo is translocated into the matrix, where the receptor is ubiquitinated and exported back to the cytosol for further rounds of matrix protein import. This review discusses the recent progress in our understanding of the peroxisomal matrix protein import and its regulation by ubiquitination events as well as the current view on the translocation mechanism of folded proteins into peroxisomes. This article is part of a Special Issue entitled: Origin and spatiotemporal dynamics of the peroxisomal endomembrane system.
Keywords: biogenesis; peroxisome; protein import; targeting; translocation; ubiquitination.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

