Role of inositol poly-phosphatases and their targets in T cell biology
- PMID: 24069021
- PMCID: PMC3779868
- DOI: 10.3389/fimmu.2013.00288
Role of inositol poly-phosphatases and their targets in T cell biology
Abstract
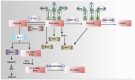
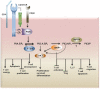
T lymphocytes play a critical role in host defense in all anatomical sites including mucosal surfaces. This not only includes the effector arm of the immune system, but also regulation of immune responses in order to prevent autoimmunity. Genetic targeting of PI3K isoforms suggests that generation of PI(3,4,5)P3 by PI3K plays a critical role in promoting effector T cell responses. Consequently, the 5'- and 3'-inositol poly-phosphatases SHIP1, SHIP2, and phosphatase and tensin homolog capable of targeting PI(3,4,5)P3 are potential genetic determinants of T cell effector functions in vivo. In addition, the 5'-inositol poly-phosphatases SHIP1 and 2 can shunt PI(3,4,5)P3 to the rare but potent signaling phosphoinositide species PI(3,4)P2 and thus these SHIP1/2, and the INPP4A/B enzymes that deplete PI(3,4)P2 may have precise roles in T cell biology to amplify or inhibit effectors of PI3K signaling that are selectively recruited to and activated by PI(3,4)P2. Here we summarize recent genetic and chemical evidence that indicates the inositol poly-phosphatases have important roles in both the effector and regulatory functions of the T cell compartment. In addition, we will discuss future genetic studies that might be undertaken to further elaborate the role of these enzymes in T cell biology as well as potential pharmaceutical manipulation of these enzymes for therapeutic purposes in disease settings where T cell function is a key in vivo target.
Keywords: INPP4; PI3K; PTEN; SHIP1; SHIP2; T cells; T lymphocytes; adoptive T cell transfer.
Figures
Similar articles
-
Phosphatidylinositol (3,4) bisphosphate-specific phosphatases and effector proteins: A distinct branch of PI3K signaling.Cell Signal. 2015 Sep;27(9):1789-98. doi: 10.1016/j.cellsig.2015.05.013. Epub 2015 May 27. Cell Signal. 2015. PMID: 26022180 Review.
-
Targeting SHIP1 and SHIP2 in Cancer.Cancers (Basel). 2021 Feb 20;13(4):890. doi: 10.3390/cancers13040890. Cancers (Basel). 2021. PMID: 33672717 Free PMC article. Review.
-
Phosphoinositide 5-phosphatase activities control cell motility in glioblastoma: Two phosphoinositides PI(4,5)P2 and PI(3,4)P2 are involved.Adv Biol Regul. 2018 Jan;67:40-48. doi: 10.1016/j.jbior.2017.09.001. Epub 2017 Sep 5. Adv Biol Regul. 2018. PMID: 28916189 Review.
-
PTEN and Other PtdIns(3,4,5)P3 Lipid Phosphatases in Breast Cancer.Int J Mol Sci. 2020 Dec 2;21(23):9189. doi: 10.3390/ijms21239189. Int J Mol Sci. 2020. PMID: 33276499 Free PMC article. Review.
-
SHIP2 signaling in normal and pathological situations: Its impact on cell proliferation.Adv Biol Regul. 2014 Jan;54:142-51. doi: 10.1016/j.jbior.2013.09.002. Epub 2013 Sep 15. Adv Biol Regul. 2014. PMID: 24091101 Review.
Cited by
-
Deciphering T Cell Immunometabolism with Activity-Based Protein Profiling.Curr Top Microbiol Immunol. 2019;420:175-210. doi: 10.1007/82_2018_124. Curr Top Microbiol Immunol. 2019. PMID: 30128827 Free PMC article. Review.
-
A small-molecule inhibitor of SHIP1 reverses age- and diet-associated obesity and metabolic syndrome.JCI Insight. 2016 Jul 21;1(11):e88544. doi: 10.1172/jci.insight.88544. JCI Insight. 2016. PMID: 27536730 Free PMC article.
-
Myo-inositol in autoimmune thyroiditis, and hypothyroidism.Rev Endocr Metab Disord. 2018 Dec;19(4):349-354. doi: 10.1007/s11154-018-9477-9. Rev Endocr Metab Disord. 2018. PMID: 30506520 Review.
-
Downregulation of PIK3IP1/TrIP on T cells is controlled by TCR signal strength, PKC and metalloprotease-mediated cleavage.bioRxiv [Preprint]. 2024 Oct 21:2024.04.29.591680. doi: 10.1101/2024.04.29.591680. bioRxiv. 2024. Update in: J Biol Chem. 2024 Dec;300(12):107930. doi: 10.1016/j.jbc.2024.107930. PMID: 38746242 Free PMC article. Updated. Preprint.
-
Structural and non-coding variants increase the diagnostic yield of clinical whole genome sequencing for rare diseases.Genome Med. 2023 Nov 9;15(1):94. doi: 10.1186/s13073-023-01240-0. Genome Med. 2023. PMID: 37946251 Free PMC article.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

