Allergen-specific IgG antibodies purified from mite-allergic patients sera block the IgE recognition of Dermatophagoides pteronyssinus antigens: an in vitro study
- PMID: 24069042
- PMCID: PMC3771262
- DOI: 10.1155/2013/657424
Allergen-specific IgG antibodies purified from mite-allergic patients sera block the IgE recognition of Dermatophagoides pteronyssinus antigens: an in vitro study
Abstract
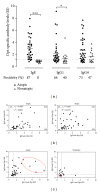
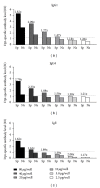
One of the purposes of specific immunotherapy (SIT) is to modulate humoral immune response against allergens with significant increases in allergen-specific IgG levels, commonly associated with blocking activity. The present study investigated in vitro blocking activity of allergen-specific IgG antibodies on IgE reactivity to Dermatophagoides pteronyssinus (Dpt) in sera from atopic patients. Dpt-specific IgG antibodies were purified by ammonium sulfate precipitation followed by protein-G affinity chromatography. Purity was checked by SDS-PAGE and immunoreactivity by slot-blot and immunoblot assays. The blocking activity was evaluated by inhibition ELISA. The electrophoretic profile of the ammonium sulfate precipitated fraction showed strongly stained bands in ligand fraction after chromatography, compatible with molecular weight of human whole IgG molecule. The purity degree was confirmed by detecting strong immunoreactivity to IgG, negligible to IgA, and no reactivity to IgE and IgM. Dpt-specific IgG fraction was capable of significantly reducing levels of IgE anti-Dpt, resulting in 35%-51% inhibition of IgE reactivity to Dpt in atopic patients sera. This study showed that allergen-specific IgG antibodies purified from mite-allergic patients sera block the IgE recognition of Dermatophagoides pteronyssinus antigens. This approach reinforces that intermittent measurement of serum allergen-specific IgG antibodies will be an important objective laboratorial parameter that will help specialists to follow their patients under SIT.
Figures



References
-
- Calderón MA, Casale TB, Togias A, Bousquet J, Durham SR, Demoly P. Allergen-specific immunotherapy for respiratory allergies: from meta-analysis to registration and beyond. Journal of Allergy and Clinical Immunology. 2011;127(1):30–38. - PubMed
-
- Tovey ER, Chapman MD, Platts-Mills TAE. Mite faeces are a major source of house dust allergens. Nature. 1981;289(5798):592–593. - PubMed
-
- Pawankar R, Canonica GW, Holgate ST, Lockey RF. Allergic diseases and asthma: a major global health concern. Current Opinion in Allergy and Clinical Immunology. 2012;12(1):39–41. - PubMed
-
- Larché M, Akdis CA, Valenta R. Immunological mechanisms of allergen-specific immunotherapy. Nature Reviews Immunology. 2006;6(10):761–771. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

