Dangguijakyak-San Protects against 1-Methyl-4-phenyl-1,2,3,6,-tetrahydropyridine-Induced Neuronal Damage via Anti-Inflammatory Action
- PMID: 24069062
- PMCID: PMC3773428
- DOI: 10.1155/2013/976270
Dangguijakyak-San Protects against 1-Methyl-4-phenyl-1,2,3,6,-tetrahydropyridine-Induced Neuronal Damage via Anti-Inflammatory Action
Abstract
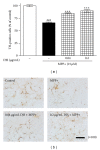
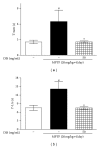
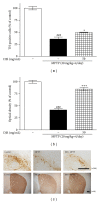
Dangguijakyak-san (DJS), a famous traditional Korean multiherbal medicine, has been used to treat gynecological and neuro-associated disease. Recent studies demonstrated that DJS has multiple bioactivities including neuroprotection. In the present study, we were to investigate the effect of DJS and its mechanism in an in vitro and in vivo model of Parkinson's disease (PD). In primary mesencephalic culture system, DJS attenuated the dopaminergic cell damage induced by 1-methyl-4-phenylpyridine toxicity, and it inhibited production of inflammatory factors such as tumor necrosis factor α (TNF- α ), nitric oxide (NO), and activation of microglial cells. Then, we confirmed the effect of DJS in a mouse PD model induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). In the pole test, DJS at 50 mg/kg/day for 5 days showed increase of motor activity showing shortened time to turn and locomotor activity compared with the MPTP only treated mice. In addition, DJS significantly protected nigrostriatal dopaminergic neuron from MPTP stress. Moreover, DJS showed inhibition of gliosis in the substantia nigra pars compacta. These results have therapeutic implications for DJS in the treatment of PD via anti-inflammatory effects.
Figures



Similar articles
-
Dangguijakyak-san protects dopamine neurons against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurotoxicity under postmenopausal conditions.J Ethnopharmacol. 2012 Feb 15;139(3):883-8. doi: 10.1016/j.jep.2011.12.015. Epub 2011 Dec 13. J Ethnopharmacol. 2012. PMID: 22178176
-
Acacetin protects dopaminergic cells against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neuroinflammation in vitro and in vivo.Biol Pharm Bull. 2012;35(8):1287-94. doi: 10.1248/bpb.b12-00127. Biol Pharm Bull. 2012. PMID: 22863927
-
Combining nitric oxide release with anti-inflammatory activity preserves nigrostriatal dopaminergic innervation and prevents motor impairment in a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson's disease.J Neuroinflammation. 2010 Nov 23;7:83. doi: 10.1186/1742-2094-7-83. J Neuroinflammation. 2010. PMID: 21092260 Free PMC article.
-
Neuroprotective effect of ghrelin in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson's disease by blocking microglial activation.Neurotox Res. 2009 May;15(4):332-47. doi: 10.1007/s12640-009-9037-x. Epub 2009 Mar 17. Neurotox Res. 2009. PMID: 19384567
-
The intranasal administration of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP): a new rodent model to test palliative and neuroprotective agents for Parkinson's disease.Curr Pharm Des. 2011;17(5):489-507. doi: 10.2174/138161211795164095. Curr Pharm Des. 2011. PMID: 21375482 Review.
Cited by
-
A narrative review of potential neural repair poststroke: Decoction of Chinese angelica and peony in regulating microglia polarization through the neurosteroid pathway.Brain Circ. 2023 Dec 21;10(1):5-10. doi: 10.4103/bc.bc_45_23. eCollection 2024 Jan-Mar. Brain Circ. 2023. PMID: 38655444 Free PMC article. Review.
-
Danggwijagyaksan for climacteric syndrome in peri- and postmenopausal women with a blood-deficiency dominant pattern: study protocol for a randomized, double-blind, placebo-controlled pilot trial.Trials. 2018 Jan 15;19(1):41. doi: 10.1186/s13063-018-2443-8. Trials. 2018. PMID: 29335018 Free PMC article.
-
Dangguijihwang-tang and Dangguijakyak-san Prevent Menopausal Symptoms and Dangguijihwang-tang Prevents Articular Cartilage Deterioration in Ovariectomized Obese Rats with Monoiodoacetate-Induced Osteoarthritis.Evid Based Complement Alternat Med. 2017;2017:5658681. doi: 10.1155/2017/5658681. Epub 2017 Oct 24. Evid Based Complement Alternat Med. 2017. PMID: 29348767 Free PMC article.
-
Dangguijakyak-san ameliorates memory deficits in ovariectomized mice by upregulating hippocampal estrogen synthesis.BMC Complement Altern Med. 2017 Nov 25;17(1):501. doi: 10.1186/s12906-017-2015-6. BMC Complement Altern Med. 2017. PMID: 29178947 Free PMC article.
-
Effects of Brain Factor‑7® against motor deficit and oxidative stress in a mouse model of MPTP‑induced Parkinson's disease.Exp Ther Med. 2022 Aug 24;24(4):635. doi: 10.3892/etm.2022.11572. eCollection 2022 Oct. Exp Ther Med. 2022. PMID: 36160902 Free PMC article.
References
-
- Paulus W, Jellinger K. The neuropathologic basis of different clinical subgroups of Parkinson’s disease. Journal of Neuropathology and Experimental Neurology. 1991;50(6):743–755. - PubMed
-
- Jenner P, Hunot H, Olanow O, et al. Oxidative stress in Parkinson’s disease. Annals of Neurology. 2003;53(supplement 3):S26–S38. - PubMed
-
- Schapira AH. Mitochondria in the aetiology and pathogenesis of Parkinson’s disease. The Lancet Neurology. 2008;7(1):97–109. - PubMed
-
- Olanow CW, Tatton WG. Etiology and pathogenesis of Parkinson’s disease. Annual Review of Neuroscience. 1999;22:123–144. - PubMed
-
- Orth M, Schapira AHV. Mitochondria and degenerative disorders. American Journal of Medical Genetics. 2001;106(1):27–36. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

