The autophagy inhibitor verteporfin moderately enhances the antitumor activity of gemcitabine in a pancreatic ductal adenocarcinoma model
- PMID: 24069069
- PMCID: PMC3781989
- DOI: 10.7150/jca.7030
The autophagy inhibitor verteporfin moderately enhances the antitumor activity of gemcitabine in a pancreatic ductal adenocarcinoma model
Abstract
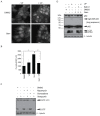
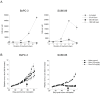
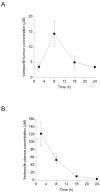
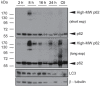
Pancreatic ductal adenocarcinoma (PDAC) is highly resistant to chemotherapy. It has been described as requiring elevated autophagy for growth and inhibiting autophagy has been proposed as a treatment strategy. To date, all preclinical reports and clinical trials investigating pharmacological inhibition of autophagy have used chloroquine or hydroxychloroquine, which interfere with lysosomal function and block autophagy at a late stage. Verteporfin is a newly discovered autophagy inhibitor that blocks autophagy at an early stage by inhibiting autophagosome formation. Here we report that PDAC cell lines show variable sensitivity to verteporfin in vitro, suggesting cell-line specific autophagy dependence. Using image-based and molecular analyses, we show that verteporfin inhibits autophagy stimulated by gemcitabine, the current standard treatment for PDAC. Pharmacokinetic and efficacy studies in a BxPC-3 xenograft mouse model demonstrated that verteporfin accumulated in tumors at autophagy-inhibiting levels and inhibited autophagy in vivo, but did not reduce tumor volume or increase survival as a single agent. In combination with gemcitabine verteporfin moderately reduced tumor growth and enhanced survival compared to gemcitabine alone. While our results do not uphold the premise that autophagy inhibition might be widely effective against PDAC as a single-modality treatment, they do support autophagy inhibition as an approach to sensitize PDAC to gemcitabine.
Keywords: autophagy; chemosensitization; gemcitabine; pancreatic cancer; verteporfin.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures

Similar articles
-
ATG4B Inhibitor UAMC-2526 Potentiates the Chemotherapeutic Effect of Gemcitabine in a Panc02 Mouse Model of Pancreatic Ductal Adenocarcinoma.Front Oncol. 2021 Nov 18;11:750259. doi: 10.3389/fonc.2021.750259. eCollection 2021. Front Oncol. 2021. PMID: 34868951 Free PMC article.
-
Niraparib perturbs autophagosome-lysosome fusion in pancreatic ductal adenocarcinoma and exhibits anticancer potential against gemcitabine-resistant PDAC.Transl Oncol. 2025 Jan;51:102206. doi: 10.1016/j.tranon.2024.102206. Epub 2024 Nov 27. Transl Oncol. 2025. PMID: 39603206 Free PMC article.
-
MicroRNA-410-3p attenuates gemcitabine resistance in pancreatic ductal adenocarcinoma by inhibiting HMGB1-mediated autophagy.Oncotarget. 2017 Nov 18;8(64):107500-107512. doi: 10.18632/oncotarget.22494. eCollection 2017 Dec 8. Oncotarget. 2017. PMID: 29296182 Free PMC article.
-
Unraveling the complexity of autophagy: Potential therapeutic applications in Pancreatic Ductal Adenocarcinoma.Semin Cancer Biol. 2015 Dec;35:11-9. doi: 10.1016/j.semcancer.2015.09.011. Epub 2015 Sep 25. Semin Cancer Biol. 2015. PMID: 26408419 Review.
-
Therapeutic Targeting of Autophagy in Pancreatic Ductal Adenocarcinoma.Front Pharmacol. 2021 Nov 30;12:751568. doi: 10.3389/fphar.2021.751568. eCollection 2021. Front Pharmacol. 2021. PMID: 34916936 Free PMC article. Review.
Cited by
-
ATG4B Inhibitor UAMC-2526 Potentiates the Chemotherapeutic Effect of Gemcitabine in a Panc02 Mouse Model of Pancreatic Ductal Adenocarcinoma.Front Oncol. 2021 Nov 18;11:750259. doi: 10.3389/fonc.2021.750259. eCollection 2021. Front Oncol. 2021. PMID: 34868951 Free PMC article.
-
The Hippo Pathway Transducers YAP1/TEAD Induce Acquired Resistance to Trastuzumab in HER2-Positive Breast Cancer.Cancers (Basel). 2020 Apr 29;12(5):1108. doi: 10.3390/cancers12051108. Cancers (Basel). 2020. PMID: 32365528 Free PMC article.
-
Hippo-YAP1 Is a Prognosis Marker and Potentially Targetable Pathway in Advanced Gallbladder Cancer.Cancers (Basel). 2020 Mar 25;12(4):778. doi: 10.3390/cancers12040778. Cancers (Basel). 2020. PMID: 32218280 Free PMC article.
-
Regulation and function of autophagy in pancreatic cancer.Autophagy. 2021 Nov;17(11):3275-3296. doi: 10.1080/15548627.2020.1847462. Epub 2020 Nov 20. Autophagy. 2021. PMID: 33161807 Free PMC article. Review.
-
Autophagy in cancer.F1000Prime Rep. 2015 Feb 3;7:18. doi: 10.12703/P7-18. eCollection 2015. F1000Prime Rep. 2015. PMID: 25750736 Free PMC article. Review.
References
-
- Mizushima N. Physiological functions of autophagy. Curr Top Microbiol Immunol. 2009;335:71–84. - PubMed
-
- Weidberg H, Shvets E, Elazar Z. Biogenesis and cargo selectivity of autophagosomes. Ann Rev Biochem. 2011;80:125–56. - PubMed
-
- Apel A, Herr I, Schwarz H, Rodemann HP, Mayer A. Blocked autophagy sensitizes resistant carcinoma cells to radiation therapy. Cancer Res. 2008;68:1485–94. - PubMed

