Pneumococcal polysaccharide abrogates conjugate-induced germinal center reaction and depletes antibody secreting cell pool, causing hyporesponsiveness
- PMID: 24069152
- PMCID: PMC3771989
- DOI: 10.1371/journal.pone.0072588
Pneumococcal polysaccharide abrogates conjugate-induced germinal center reaction and depletes antibody secreting cell pool, causing hyporesponsiveness
Abstract
Background: Plain pneumococcal polysaccharide (PPS) booster administered during second year of life has been shown to cause hyporesponsiveness. We assessed the effects of PPS booster on splenic memory B cell responses and persistence of PPS-specific long-lived plasma cells in the bone marrow (BM).
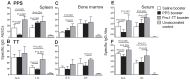
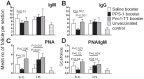
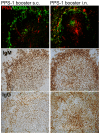
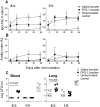
Methods: Neonatal mice were primed subcutanously (s.c.) or intranasally (i.n.) with pneumococcal conjugate (Pnc1-TT) and the adjuvant LT-K63, and boosted with PPS+LT-K63 or saline 1, 2 or 3 times with 16 day intervals. Seven days after each booster, spleens were removed, germinal centers (GC), IgM(+), IgG(+) follicles and PPS-specific antibody secreting cells (AbSC) in spleen and BM enumerated.
Results: PPS booster s.c., but not i.n., compromised the Pnc1-TT-induced PPS-specific Abs by abrogating the Pnc1-TT-induced GC reaction and depleting PPS-specific AbSCs in spleen and limiting their homing to the BM. There was no difference in the frequency of PPS-specific AbSCs in spleen and BM between mice that received 1, 2 or 3 PPS boosters s.c.. Repeated PPS+LT-K63 booster i.n. reduced the frequency of PPS-specific IgG(+) AbSCs in BM.
Conclusions: PPS booster-induced hyporesponsiveness is caused by abrogation of conjugate-induced GC reaction and depletion of PPS-specific IgG(+) AbSCs resulting in no homing of new PPS-specific long-lived plasma cells to the BM or survival. These results should be taken into account in design of vaccination schedules where polysaccharides are being considered.
Conflict of interest statement
Figures


Similar articles
-
Adjuvants Enhance the Induction of Germinal Center and Antibody Secreting Cells in Spleen and Their Persistence in Bone Marrow of Neonatal Mice.Front Immunol. 2019 Sep 26;10:2214. doi: 10.3389/fimmu.2019.02214. eCollection 2019. Front Immunol. 2019. PMID: 31616417 Free PMC article.
-
The adjuvant LT-K63 can restore delayed maturation of follicular dendritic cells and poor persistence of both protein- and polysaccharide-specific antibody-secreting cells in neonatal mice.J Immunol. 2012 Aug 1;189(3):1265-73. doi: 10.4049/jimmunol.1200761. Epub 2012 Jul 2. J Immunol. 2012. PMID: 22753937 Free PMC article.
-
The advantage of mucosal immunization for polysaccharide-specific memory responses in early life.Eur J Immunol. 2005 Apr;35(4):1037-45. doi: 10.1002/eji.200425850. Eur J Immunol. 2005. PMID: 15756644
-
Intranasal immunization with pneumococcal conjugate vaccines with LT-K63, a nontoxic mutant of heat-Labile enterotoxin, as adjuvant rapidly induces protective immunity against lethal pneumococcal infections in neonatal mice.Infect Immun. 2002 Mar;70(3):1443-52. doi: 10.1128/IAI.70.3.1443-1452.2002. Infect Immun. 2002. PMID: 11854231 Free PMC article.
-
Protective levels of polysaccharide-specific maternal antibodies may enhance the immune response elicited by pneumococcal conjugates in neonatal and infant mice.Infect Immun. 2005 Feb;73(2):956-64. doi: 10.1128/IAI.73.2.956-964.2005. Infect Immun. 2005. PMID: 15664938 Free PMC article.
Cited by
-
Ontogeny of early life immunity.Trends Immunol. 2014 Jul;35(7):299-310. doi: 10.1016/j.it.2014.04.007. Epub 2014 May 28. Trends Immunol. 2014. PMID: 24880460 Free PMC article. Review.
-
Pneumococcal Vaccination in High-Risk Individuals: Are We Doing It Right?Clin Vaccine Immunol. 2016 May 6;23(5):388-395. doi: 10.1128/CVI.00721-15. Print 2016 May. Clin Vaccine Immunol. 2016. PMID: 27009210 Free PMC article. Review.
-
The role of antigen availability during B cell induction and its effect on sustained memory and antibody production after infection and vaccination-lessons learned from the SARS-CoV-2 pandemic.Clin Exp Immunol. 2022 Dec 31;210(3):273-282. doi: 10.1093/cei/uxac113. Clin Exp Immunol. 2022. PMID: 36480298 Free PMC article.
-
No long-term evidence of hyporesponsiveness after use of pneumococcal conjugate vaccine in children previously immunized with pneumococcal polysaccharide vaccine.J Allergy Clin Immunol. 2016 Jun;137(6):1772-1779.e11. doi: 10.1016/j.jaci.2015.12.1303. Epub 2016 Jan 26. J Allergy Clin Immunol. 2016. PMID: 26825000 Free PMC article. Clinical Trial.
-
LT-K63 Enhances B Cell Activation and Survival Factors in Neonatal Mice That Translates Into Long-Lived Humoral Immunity.Front Immunol. 2020 Oct 23;11:527310. doi: 10.3389/fimmu.2020.527310. eCollection 2020. Front Immunol. 2020. PMID: 33193301 Free PMC article.
References
-
- WHO (2012) Pneumococcal conjugate vaccine for childhood immunization – WHO position paper 2012. Wkly Epidemiol Rec 87: 129–144. - PubMed
-
- Douglas RM, Paton JC, Duncan SJ, Hansman DJ (1983) Antibody response to pneumococcal vaccination in children younger than five years of age. J Infect Dis 148: 131–137. - PubMed
-
- Granat SM, Mia Z, Ollgren J, Herva E, Das M, et al. (2007) Longitudinal study on pneumococcal carriage during the first year of life in Bangladesh. Pediatr Infect Dis J 26: 319–324. - PubMed
-
- Simell B, Auranen K, Kayhty H, Goldblatt D, Dagan R, et al. (2013) The fundamental link between pneumococcal carriage and disease. Expert Rev Vaccines 11: 841–855. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

