The ral exchange factor rgl2 promotes cardiomyocyte survival and inhibits cardiac fibrosis
- PMID: 24069211
- PMCID: PMC3775766
- DOI: 10.1371/journal.pone.0073599
The ral exchange factor rgl2 promotes cardiomyocyte survival and inhibits cardiac fibrosis
Abstract
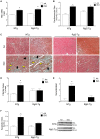
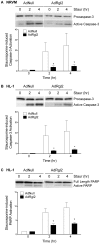
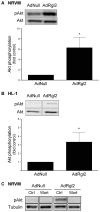
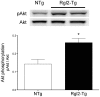
Cardiomyocytes compensate to acute cardiac stress by increasing in size and contractile function. However, prolonged stress leads to a decompensated response characterized by cardiomyocyte death, tissue fibrosis and loss of cardiac function. Identifying approaches to inhibit this transition to a decompensated response may reveal important targets for treating heart failure. The Ral guanine nucleotide disassociation (RalGDS) proteins are Ras-interacting proteins that are upregulated by hypertrophic stimuli. The Ral guanine nucleotide dissociation stimulator-like 2 (Rgl2) is a member of the RalGDS family that modulates expression of hypertrophic genes in cardiomyocytes. However, the pathophysiologic consequence of increased Rgl2 expression in cardiomyoctyes remains unclear. To evaluate the effect of increasing Rgl2 activity in the heart, transgenic mice with cardiac-targeted over-expression of Rgl2 were generated. Although Ral activation was increased, there were no apparent morphologic or histological differences between the hearts of Rgl2 transgenic and nontransgenic mice indicating that increased Rgl2 expression had no effect on basal cardiac phenotype. To determine if Rgl2 modulates the cardiac response to stress, mice were infused with the ß-adrenergic receptor agonist, isoproterenol. Isoproterenol infusion increased heart mass in both Rgl2 transgenic and nontransgenic mice. However, unlike nontransgenic mice, Rgl2 transgenic mice showed no morphologic evidence of cardiomyocyte damage or increased cardiac fibrosis following isoproterenol infusion. Increased Rgl2 expression in cultured cardiomyocytes stimulated Ral activation and inhibited staurosporine-induced apoptosis via increased activation of PI3-kinase. Activation of the PI3-kinase signaling pathway was confirmed in hearts isolated from Rgl2 transgenic mice. Increased expression and function of Rgl2 in cardiomyocytes promotes activation of the PI3-kinase signaling cascade and protects from carciomyocyte death and pathologic cardiac fibrosis. Taken further, these results suggest that Rgl2 upregulation in hypertrophic hearts may be a protetive mechanism, and that Rgl2 may be a novel therapeutic target in treating heart disease.
Conflict of interest statement
Figures




Similar articles
-
G-protein binding features and regulation of the RalGDS family member, RGL2.Biochem J. 2008 Oct 1;415(1):145-54. doi: 10.1042/BJ20080255. Biochem J. 2008. PMID: 18540861
-
Aberrant overexpression of the Rgl2 Ral small GTPase-specific guanine nucleotide exchange factor promotes pancreatic cancer growth through Ral-dependent and Ral-independent mechanisms.J Biol Chem. 2010 Nov 5;285(45):34729-40. doi: 10.1074/jbc.M110.116756. Epub 2010 Aug 27. J Biol Chem. 2010. PMID: 20801877 Free PMC article.
-
RalGDS-dependent cardiomyocyte autophagy is required for load-induced ventricular hypertrophy.J Mol Cell Cardiol. 2013 Jun;59:128-38. doi: 10.1016/j.yjmcc.2013.02.015. Epub 2013 Mar 6. J Mol Cell Cardiol. 2013. PMID: 23473774 Free PMC article.
-
Ral GDP dissociation stimulator and Ral GTPase are involved in myocardial hypertrophy.Hypertension. 2003 Apr;41(4):956-62. doi: 10.1161/01.HYP.0000063884.36641.63. Epub 2003 Mar 17. Hypertension. 2003. PMID: 12642511
-
RalGDS family members couple Ras to Ral signalling and that's not all.Cell Signal. 2010 Dec;22(12):1804-10. doi: 10.1016/j.cellsig.2010.05.010. Epub 2010 May 15. Cell Signal. 2010. PMID: 20478380 Review.
Cited by
-
DNA methylation patterns associated with oxidative stress in an ageing population.BMC Med Genomics. 2016 Nov 25;9(1):72. doi: 10.1186/s12920-016-0235-0. BMC Med Genomics. 2016. PMID: 27884142 Free PMC article.
-
Literature-Based Discovery to Elucidate the Biological Links between Resistant Hypertension and COVID-19.Biology (Basel). 2023 Sep 21;12(9):1269. doi: 10.3390/biology12091269. Biology (Basel). 2023. PMID: 37759668 Free PMC article.
-
Cytoprotective effects of the medicinal herb Astragalus membranaceus on lipopolysaccharide‑exposed cells.Mol Med Rep. 2018 Nov;18(5):4321-4327. doi: 10.3892/mmr.2018.9483. Epub 2018 Sep 14. Mol Med Rep. 2018. PMID: 30221731 Free PMC article.
-
The Isoforms of Ral Guanine Nucleotide Dissociation Stimulator (RalGDS) in LLC-PK1 Cells.Curr Issues Mol Biol. 2025 Jul 18;47(7):566. doi: 10.3390/cimb47070566. Curr Issues Mol Biol. 2025. PMID: 40729035 Free PMC article.
References
-
- Heineke J, Molkentin JD (2006) Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol 7: 589–600. - PubMed
-
- McMullen JR, Jennings GL (2007) Differences between pathological and physiological cardiac hypertrophy: novel therapeutic strategies to treat heart failure. Clinical and Experimental Pharmacology and Physiology 34: 255–262. - PubMed
-
- Frey N, Olson E (2003) CARDIAC HYPERTROPHY: The Good, the Bad, and the Ugly. Annual Review of Physiology 65: 45–79. - PubMed
-
- Diwan A, Dorn GW (2007) Decompensation of cardiac hypertrophy: cellular mechanisms and novel therapeutic targets. Physiology (Bethesda, Md) 22: 56–64. - PubMed
-
- Hunter JJ, Chien KR (1999) Signaling pathways for cardiac hypertrophy and failure. The New England journal of medicine 341: 1276–1283. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

