Population structure of manganese-oxidizing bacteria in stratified soils and properties of manganese oxide aggregates under manganese-complex medium enrichment
- PMID: 24069232
- PMCID: PMC3772008
- DOI: 10.1371/journal.pone.0073778
Population structure of manganese-oxidizing bacteria in stratified soils and properties of manganese oxide aggregates under manganese-complex medium enrichment
Abstract
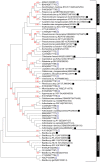
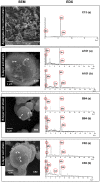
Manganese-oxidizing bacteria in the aquatic environment have been comprehensively investigated. However, little information is available about the distribution and biogeochemical significance of these bacteria in terrestrial soil environments. In this study, stratified soils were initially examined to investigate the community structure and diversity of manganese-oxidizing bacteria. Total 344 culturable bacterial isolates from all substrata exhibited Mn(II)-oxidizing activities at the range of 1 µM to 240 µM of the equivalent MnO2. The high Mn(II)-oxidizing isolates (>50 mM MnO2) were identified as the species of phyla Actinobacteria, Firmicutes and Proteobacteria. Seven novel Mn(II)-oxidizing bacterial genera (species), namely, Escherichia, Agromyces, Cellulomonas, Cupriavidus, Microbacterium, Ralstonia, and Variovorax, were revealed via comparative phylogenetic analysis. Moreover, an increase in the diversity of soil bacterial community was observed after the combined enrichment of Mn(II) and carbon-rich complex. The phylogenetic classification of the enriched bacteria represented by predominant denaturing gradient gel electrophoresis bands, was apparently similar to culturable Mn(II)-oxidizing bacteria. The experiments were further undertaken to investigate the properties of the Mn oxide aggregates formed by the bacterial isolates with high Mn(II)-oxidizing activity. Results showed that these bacteria were closely encrusted with their Mn oxides and formed regular microspherical aggregates under prolonged Mn(II) and carbon-rich medium enrichment for three weeks. The biotic oxidation of Mn(II) to Mn(III/IV) by these isolates was confirmed by kinetic examinations. X-ray diffraction assays showed the characteristic peaks of several Mn oxides and rhodochrosite from these aggregates. Leucoberbelin blue tests also verified the Mn(II)-oxidizing activity of these aggregates. These results demonstrated that Mn oxides were formed at certain amounts under the enrichment conditions, along with the formation of rhodochrosite in such aggregates. Therefore, this study provides insights into the structure and diversity of soil-borne bacterial communities in Mn(II)-oxidizing habitats and supports the contribution of soil-borne Mn(II)-oxidizing bacteria to Mn oxide mineralization in soils.
Conflict of interest statement
Figures



References
-
- Tebo BM, Bargar JR, Clement BG, Dick GJ, Murray KJ, et al. (2004) Biogenic manganese oxides: Properties and mechanisms of formation. Annu Rev Earth Pl Sc 32: 287–328.
-
- Kay JT, Conklin MH, Fuller CC, O’Day PA (2001) Processes of nickel and cobalt uptake by a manganese oxide forming sediment in Pinal Creek, globe mining district, Arizona. Environ Sci Technol 135: 4719–4725. - PubMed
-
- Tebo BM, Johnson HA, McCarthy JK, Templeton AS (2005) Geomicrobiology of manganese(II) oxidation. Trends Microbiol 13: 421–428. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

