Prognostic value of CD109+ circulating endothelial cells in recurrent glioblastomas treated with bevacizumab and irinotecan
- PMID: 24069296
- PMCID: PMC3772091
- DOI: 10.1371/journal.pone.0074345
Prognostic value of CD109+ circulating endothelial cells in recurrent glioblastomas treated with bevacizumab and irinotecan
Abstract
Background: Recent data suggest that circulating endothelial and progenitor cells (CECs and CEPs, respectively) may have predictive potential in cancer patients treated with bevacizumab, the antibody recognizing vascular endothelial growth factor (VEGF). Here we report on CECs and CEPs investigated in 68 patients affected by recurrent glioblastoma (rGBM) treated with bevacizumab and irinotecan and two Independent Datasets of rGBM patients respectively treated with bevacizumab alone (n=32, independent dataset A: IDA) and classical antiblastic chemotherapy (n=14, independent dataset B: IDB).
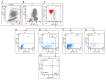
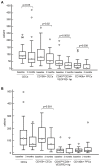
Methods: rGBM patients with KPS ≥50 were treated until progression, as defined by MRI with RANO criteria. CECs expressing CD109, a marker of tumor endothelial cells, as well as other CEC and CEP subtypes, were investigated by six-color flow cytometry.
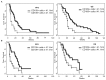
Results: A baseline count of CD109+ CEC higher than 41.1/ml (1(st) quartile) was associated with increased progression free survival (PFS; 20 versus 9 weeks, P=0.008) and overall survival (OS; 32 versus 23 weeks, P=0.03). Longer PFS (25 versus 8 weeks, P=0.02) and OS (27 versus 17 weeks, P=0.03) were also confirmed in IDA with CD109+ CECs higher than 41.1/ml but not in IDB. Patients treated with bevacizumab with or without irinotecan that were free from MRI progression after two months of treatment had significant decrease of CD109+ CECs: median PFS was 19 weeks; median OS 29 weeks. The presence of two non-contiguous lesions (distant disease) at baseline was an independent predictor of shorter PFS and OS (P<0.001).
Conclusions: Data encourage further studies on the predictive potential of CD109+ CECs in GBM patients treated with bevacizumab.
Conflict of interest statement
Figures


Similar articles
-
Prognostic value and kinetics of circulating endothelial cells in patients with recurrent glioblastoma randomised to bevacizumab plus lomustine, bevacizumab single agent or lomustine single agent. A report from the Dutch Neuro-Oncology Group BELOB trial.Br J Cancer. 2015 Jul 14;113(2):226-31. doi: 10.1038/bjc.2015.191. Epub 2015 Jun 4. Br J Cancer. 2015. PMID: 26042933 Free PMC article. Clinical Trial.
-
Early post-bevacizumab progression on contrast-enhanced MRI as a prognostic marker for overall survival in recurrent glioblastoma: results from the ACRIN 6677/RTOG 0625 Central Reader Study.Neuro Oncol. 2013 Jul;15(7):945-54. doi: 10.1093/neuonc/not049. Epub 2013 Jun 19. Neuro Oncol. 2013. PMID: 23788270 Free PMC article. Clinical Trial.
-
Parametric response maps of perfusion MRI may identify recurrent glioblastomas responsive to bevacizumab and irinotecan.PLoS One. 2014 Mar 27;9(3):e90535. doi: 10.1371/journal.pone.0090535. eCollection 2014. PLoS One. 2014. PMID: 24675671 Free PMC article.
-
Bevacizumab: a treatment option for recurrent glioblastoma multiforme.Ann Pharmacother. 2008 Oct;42(10):1486-90. doi: 10.1345/aph.1L030. Epub 2008 Sep 2. Ann Pharmacother. 2008. PMID: 18765835 Review.
-
Efficacy and safety of bevacizumab plus capecitabine and irinotecan regimen for metastatic colorectal cancer.Med Oncol. 2010 Sep;27(3):585-91. doi: 10.1007/s12032-009-9253-5. Epub 2009 Jun 13. Med Oncol. 2010. PMID: 19526201 Review.
Cited by
-
Reduced expression of CD109 in tumor-associated endothelial cells promotes tumor progression by paracrine interleukin-8 in hepatocellular carcinoma.Oncotarget. 2016 May 17;7(20):29333-45. doi: 10.18632/oncotarget.8787. Oncotarget. 2016. PMID: 27121053 Free PMC article.
-
A subpopulation of circulating endothelial cells express CD109 and is enriched in the blood of cancer patients.PLoS One. 2014 Dec 15;9(12):e114713. doi: 10.1371/journal.pone.0114713. eCollection 2014. PLoS One. 2014. PMID: 25506915 Free PMC article.
-
Bevacizumab and radiotherapy for the treatment of glioblastoma: brothers in arms or unholy alliance?Oncotarget. 2016 Jan 19;7(3):2313-28. doi: 10.18632/oncotarget.6320. Oncotarget. 2016. PMID: 26575171 Free PMC article. Review.
-
Predictive and prognostic significance of circulating endothelial cells in advanced non-small cell lung cancer patients.Tumour Biol. 2015 Nov;36(11):9031-7. doi: 10.1007/s13277-015-3657-y. Epub 2015 Jun 18. Tumour Biol. 2015. PMID: 26084612
-
Prognostic value and kinetics of circulating endothelial cells in patients with recurrent glioblastoma randomised to bevacizumab plus lomustine, bevacizumab single agent or lomustine single agent. A report from the Dutch Neuro-Oncology Group BELOB trial.Br J Cancer. 2015 Jul 14;113(2):226-31. doi: 10.1038/bjc.2015.191. Epub 2015 Jun 4. Br J Cancer. 2015. PMID: 26042933 Free PMC article. Clinical Trial.
References
-
- Iwamoto FM, Fine HA (2010) Bevacizumab for malignant gliomas. Arch Neurol 67: 285-288. doi:10.1001/archneurol.2010.11. PubMed: 20212225. - DOI - PMC - PubMed
-
- Jubb AM, Harris AL (2010) Biomarkers to predict the clinical efficacy of bevacizumab in cancer. Lancet Oncol 11: 1172-1183. doi:10.1016/S1470-2045(10)70232-1. PubMed: 21126687. - DOI - PubMed
-
- Bertolini F, Shaked Y, Mancuso P, Kerbel RS (2006) The multifaceted circulating endothelial cell in cancer: Towards marker and target identification. Nat Rev Cancer 6: 835-845. doi:10.1038/nrc1971. PubMed: 17036040. - DOI - PubMed
-
- Bertolini F, Mancuso P, Shaked Y (2011) Circulating endothelial cells as biomarkers for patients receiving bevacizumab. Lancet Oncol 12: 217-218. doi:10.1016/S1470-2045(11)70050-X. PubMed: 21376289. - DOI - PubMed
-
- Calleri A, Bono A, Bagnardi V, Quarna J, Mancuso P et al. (2009) Predictive potential of angiogenic growth factors and circulating endothelial cells in breast cancer patients receiving metronomic chemotherapy plus bevacizumab. Clin Cancer Res 15: 7652-7657. doi:10.1158/1078-0432.CCR-09-1493. PubMed: 19996223. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

