VEZT, a novel putative tumor suppressor, suppresses the growth and tumorigenicity of gastric cancer
- PMID: 24069310
- PMCID: PMC3775783
- DOI: 10.1371/journal.pone.0074409
VEZT, a novel putative tumor suppressor, suppresses the growth and tumorigenicity of gastric cancer
Abstract
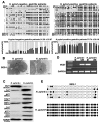
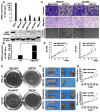
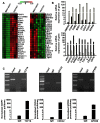
Vezatin (VEZT), an adherens junctions transmembrane protein, was identified as a putative tumor suppressor in our previous study. However, the role of VEZT in tumorigenesis remains elusive. We aimed to clarify its epigenetic regulation and biological functions in gastric cancer. In this study, we show that the expression level of VEZT is involved in lymphatic metastasis, depth of cancer invasion and TNM stage in 104 gastric cancer patients. Bisulfate sequencing polymerase chain reaction (BSP) methods showed that VEZT was hypermethylated in tissues and corresponding blood of gastric cancer patients compared with healthy controls. Helicobacter pylori (H. pylori) infection induces the methylation and silencing of VEZT in GES-1 cells. Restoring VEZT expression in MKN-45 and NCI-N87 gastric cancer cells inhibited growth, invasion and tumorigenesis in vitro and in vivo. Global microarray analysis was applied to analyze the molecular basis of the biological functions of VEZT after VEZT transfection combined with real-time PCR and chromatin immunoprecipitation assay. G protein-coupled receptor 56(GPR56), cell growth, cell division cycle 42(CDC42), migration/invasion and transcription factor 19(TCF19), cell cycle progression, were identified as direct VEZT target genes. TCF19, a novel target of VEZT, was functionally validated. Overexpression of TCF19 in MKN-45 cells increased cell cycle progress and growth ability. This study provides novel insight into the regulation of the VEZT gene, which could represent a potential target for therapeutic anti-cancer strategies.
Conflict of interest statement
Figures


Similar articles
-
VEZT as a novel independent prognostic factor in gastric cancer.Cancer Biomark. 2015;15(4):375-80. doi: 10.3233/CBM-150476. Cancer Biomark. 2015. PMID: 25792470
-
Down-regulation of VEZT gene expression in human gastric cancer involves promoter methylation and miR-43c.Biochem Biophys Res Commun. 2011 Jan 14;404(2):622-7. doi: 10.1016/j.bbrc.2010.12.026. Epub 2010 Dec 13. Biochem Biophys Res Commun. 2011. PMID: 21156161
-
Up-regulation of VEZT by small activating RNA inhibits the proliferation, invasion and migration of gastric cancer cells.Biochem Biophys Res Commun. 2017 Jan 22;482(4):542-548. doi: 10.1016/j.bbrc.2016.11.071. Epub 2016 Nov 14. Biochem Biophys Res Commun. 2017. PMID: 27856244
-
Inhibition of gastric carcinogenesis by the hormone gastrin is mediated by suppression of TFF1 epigenetic silencing.Gastroenterology. 2011 Mar;140(3):879-91. doi: 10.1053/j.gastro.2010.11.037. Epub 2010 Nov 25. Gastroenterology. 2011. PMID: 21111741 Free PMC article.
-
Gene methylation in gastric cancer.Clin Chim Acta. 2013 Sep 23;424:53-65. doi: 10.1016/j.cca.2013.05.002. Epub 2013 May 10. Clin Chim Acta. 2013. PMID: 23669186 Review.
Cited by
-
Immunotherapeutic Value of Transcription Factor 19 (TCF19) Associated with Renal Clear Cell Carcinoma: A Comprehensive Analysis of 33 Human Cancer Cases.J Oncol. 2022 Sep 6;2022:1488165. doi: 10.1155/2022/1488165. eCollection 2022. J Oncol. 2022. PMID: 36111242 Free PMC article.
-
Prognostic significance of X-linked inhibitor of apoptosis protein in patients with gastrointestinal tract cancers: A meta-analysis.Medicine (Baltimore). 2020 Feb;99(9):e18497. doi: 10.1097/MD.0000000000018497. Medicine (Baltimore). 2020. PMID: 32118702 Free PMC article.
-
Helicobacter pylori-induced inflammation and epigenetic changes during gastric carcinogenesis.World J Gastroenterol. 2015 Dec 7;21(45):12742-56. doi: 10.3748/wjg.v21.i45.12742. World J Gastroenterol. 2015. PMID: 26668499 Free PMC article. Review.
-
Microbiota-driven epigenetic modifications in gastrointestinal cancer: Implications for pathogenesis and therapeutic strategies.World J Microbiol Biotechnol. 2025 Jul 28;41(8):288. doi: 10.1007/s11274-025-04457-w. World J Microbiol Biotechnol. 2025. PMID: 40719959 Review.
-
Helicobacter pylori-induced DNA Methylation as an Epigenetic Modulator of Gastric Cancer: Recent Outcomes and Future Direction.Pathogens. 2019 Feb 13;8(1):23. doi: 10.3390/pathogens8010023. Pathogens. 2019. PMID: 30781778 Free PMC article. Review.
References
-
- Forman D, Burley VJ (2006) Gastric cancer: global pattern of the disease and an overview of environmental risk factors. Best Pract Res Clin Gastroenterol 20: 633-649. doi:10.1016/j.bpg.2006.04.008. PubMed: 16997150. - DOI - PubMed
-
- Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S et al. (2001) Helicobacter pylori infection and the development of gastric cancer. N Engl J Med 345: 784-789. doi:10.1056/NEJMoa001999. PubMed: 11556297. - DOI - PubMed
-
- Becker KF, Keller G, Hoefler H (2000) The use of molecular biology in diagnosis and prognosis of gastric cancer. Surg Oncol 9: 5-11. doi:10.1016/S0960-7404(00)00016-5. PubMed: 11525306. - DOI - PubMed
-
- Kang GH, Lee S, Kim JS, Jung HY (2003) Profile of aberrant CpG island methylation along multistep gastric carcinogenesis. Lab Invest 83: 519-526. doi:10.1097/01.LAB.0000064704.53132.65. PubMed: 12695555. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

