Mu opioid receptors on primary afferent nav1.8 neurons contribute to opiate-induced analgesia: insight from conditional knockout mice
- PMID: 24069332
- PMCID: PMC3771900
- DOI: 10.1371/journal.pone.0074706
Mu opioid receptors on primary afferent nav1.8 neurons contribute to opiate-induced analgesia: insight from conditional knockout mice
Abstract
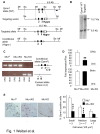
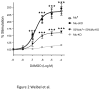
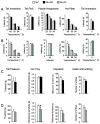
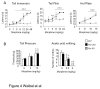
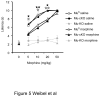
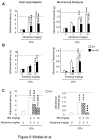
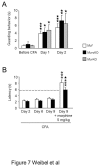
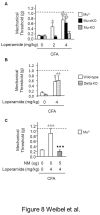
Opiates are powerful drugs to treat severe pain, and act via mu opioid receptors distributed throughout the nervous system. Their clinical use is hampered by centrally-mediated adverse effects, including nausea or respiratory depression. Here we used a genetic approach to investigate the potential of peripheral mu opioid receptors as targets for pain treatment. We generated conditional knockout (cKO) mice in which mu opioid receptors are deleted specifically in primary afferent Nav1.8-positive neurons. Mutant animals were compared to controls for acute nociception, inflammatory pain, opiate-induced analgesia and constipation. There was a 76% decrease of mu receptor-positive neurons and a 60% reduction of mu-receptor mRNA in dorsal root ganglia of cKO mice. Mutant mice showed normal responses to heat, mechanical, visceral and chemical stimuli, as well as unchanged morphine antinociception and tolerance to antinociception in models of acute pain. Inflammatory pain developed similarly in cKO and controls mice after Complete Freund's Adjuvant. In the inflammation model, however, opiate-induced (morphine, fentanyl and loperamide) analgesia was reduced in mutant mice as compared to controls, and abolished at low doses. Morphine-induced constipation remained intact in cKO mice. We therefore genetically demonstrate for the first time that mu opioid receptors partly mediate opiate analgesia at the level of Nav1.8-positive sensory neurons. In our study, this mechanism operates under conditions of inflammatory pain, but not nociception. Previous pharmacology suggests that peripheral opiates may be clinically useful, and our data further demonstrate that Nav1.8 neuron-associated mu opioid receptors are feasible targets to alleviate some forms of persistent pain.
Conflict of interest statement
Figures

Similar articles
-
μ-Opioid receptors in primary sensory neurons are essential for opioid analgesic effect on acute and inflammatory pain and opioid-induced hyperalgesia.J Physiol. 2019 Mar;597(6):1661-1675. doi: 10.1113/JP277428. Epub 2019 Jan 16. J Physiol. 2019. PMID: 30578671 Free PMC article.
-
Genetic ablation of delta opioid receptors in nociceptive sensory neurons increases chronic pain and abolishes opioid analgesia.Pain. 2011 Jun;152(6):1238-1248. doi: 10.1016/j.pain.2010.12.031. Epub 2011 Feb 3. Pain. 2011. PMID: 21295407
-
μ-Opioid receptors in primary sensory neurons are involved in supraspinal opioid analgesia.Brain Res. 2020 Feb 15;1729:146623. doi: 10.1016/j.brainres.2019.146623. Epub 2019 Dec 24. Brain Res. 2020. PMID: 31881186 Free PMC article.
-
On the Role of Peripheral Sensory and Gut Mu Opioid Receptors: Peripheral Analgesia and Tolerance.Molecules. 2020 May 26;25(11):2473. doi: 10.3390/molecules25112473. Molecules. 2020. PMID: 32466522 Free PMC article. Review.
-
Functional relevance of μ-δ opioid receptor heteromerization: a role in novel signaling and implications for the treatment of addiction disorders: from a symposium on new concepts in mu-opioid pharmacology.Drug Alcohol Depend. 2012 Mar 1;121(3):167-72. doi: 10.1016/j.drugalcdep.2011.10.025. Epub 2011 Nov 23. Drug Alcohol Depend. 2012. PMID: 22115888 Free PMC article. Review.
Cited by
-
Opioids modulate an emergent rhythmogenic process to depress breathing.Elife. 2019 Dec 16;8:e50613. doi: 10.7554/eLife.50613. Elife. 2019. PMID: 31841107 Free PMC article.
-
Delta opioid receptors on nociceptive sensory neurons mediate peripheral endogenous analgesia in colitis.J Neuroinflammation. 2022 Jan 6;19(1):7. doi: 10.1186/s12974-021-02352-3. J Neuroinflammation. 2022. PMID: 34991641 Free PMC article.
-
The Guts of the Opioid Crisis.Physiology (Bethesda). 2021 Sep 1;36(5):315-323. doi: 10.1152/physiol.00014.2021. Physiology (Bethesda). 2021. PMID: 34431418 Free PMC article.
-
Endogenous Opioid Signaling in the Mouse Retina Modulates Pupillary Light Reflex.Int J Mol Sci. 2021 Jan 8;22(2):554. doi: 10.3390/ijms22020554. Int J Mol Sci. 2021. PMID: 33429857 Free PMC article.
-
Mu opioid receptors gate the locus coeruleus pain generator.bioRxiv [Preprint]. 2024 Aug 28:2023.10.20.562785. doi: 10.1101/2023.10.20.562785. bioRxiv. 2024. PMID: 37961541 Free PMC article. Preprint.
References
-
- Le Merrer J, Becker JA, Befort K, Kieffer BL (2009) Reward processing by the opioid system in the brain. Physiol Rev 89: 1379-1412. doi:10.1152/physrev.00005.2009. PubMed: 19789384. - DOI - PMC - PubMed
-
- Mansour A, Fox CA, Akil H, Watson SJ (1995) Opioid-receptor mRNA expression in the rat CNS: anatomical and functional implications. Trends Neurosci 18: 22-29. doi:10.1016/0166-2236(95)93946-U. PubMed: 7535487. - DOI - PubMed
-
- Stein C, Machelska H (2011) Modulation of peripheral sensory neurons by the immune system: implications for pain therapy. Pharmacol Rev 63: 860-881. doi:10.1124/pr.110.003145. PubMed: 21969325. - DOI - PubMed
-
- Ossipov MH, Dussor GO, Porreca F (2010) Central modulation of pain. J Clin Invest 120: 3779-3787. doi:10.1172/JCI43766. PubMed: 21041960. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

