Behavioral and metabolic effects of the atypical antipsychotic ziprasidone on the nematode Caenorhabditis elegans
- PMID: 24069346
- PMCID: PMC3777939
- DOI: 10.1371/journal.pone.0074780
Behavioral and metabolic effects of the atypical antipsychotic ziprasidone on the nematode Caenorhabditis elegans
Abstract
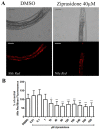
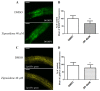
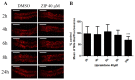
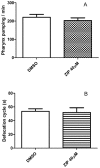
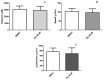
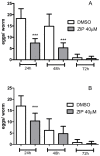
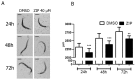
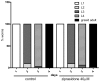
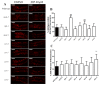
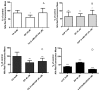
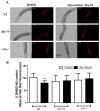
Atypical antipsychotics are associated with metabolic syndrome, primarily associated with weight gain. The effects of Ziprasidone, an atypical antipsychotic, on metabolic syndrome has yet to be evaluated. Here in, we evaluated lipid accumulation and behavioral changes in a new experimental model, the nematode Caenorhabditis elegans (C. elegans). Behavioral parameters in the worms were evaluated 24 h after Ziprasidone treatment. Subsequently, lipid accumulation was examined using Nile red, LipidTox green and BODIPY labeling. Ziprasidone at 40 µM for 24 h effectively decreased the fluorescence labeling of all markers in intestinal cells of C. elegans compared to control (0.16% dimethyl sulfoxide). Ziprasidone did not alter behaviors related to energetic balance, such as pharynx pumping, defecation cycles and movement. There was, however, a reduction in egg-production, egg-laying and body-length in nematodes exposed to Ziprasidone without any changes in the progression of larval stages. The serotoninergic pathway did not appear to modulate Ziprasidone's effects on Nile red fluorescence. Additionally, Ziprasidone did not alter lipid accumulation in daf-16 or crh-1 deletion mutants (orthologous of the transcription factors DAF-16 and CREB, respectively). These results suggest that Ziprasidone alters reproductive behavior, morphology and lipid reserves in the intestinal cells of C. elegans. Our results highlight that the DAF-16 and CREB transcription factors are essential for Ziprasidone-induced fat store reduction.
Conflict of interest statement
Figures
Similar articles
-
Impact of nuclear distribution element genes in the typical and atypical antipsychotics effects on nematode Caenorhabditis elegans: Putative animal model for studying the pathways correlated to schizophrenia.Prog Neuropsychopharmacol Biol Psychiatry. 2019 Jun 8;92:19-30. doi: 10.1016/j.pnpbp.2018.12.010. Epub 2018 Dec 19. Prog Neuropsychopharmacol Biol Psychiatry. 2019. PMID: 30578843
-
Using oral ziprasidone effectively: the food effect and dose-response.Adv Ther. 2009 Aug;26(8):739-48. doi: 10.1007/s12325-009-0055-0. Epub 2009 Aug 8. Adv Ther. 2009. PMID: 19669631 Review.
-
Investigation into the effects of the novel antipsychotic ziprasidone on weight gain and reproductive function in female rats.Behav Brain Res. 2005 May 28;160(2):338-43. doi: 10.1016/j.bbr.2004.12.015. Epub 2005 Jan 17. Behav Brain Res. 2005. PMID: 15863230
-
The effect of ziprasidone on body weight and energy expenditure in female rats.Metabolism. 2012 Jun;61(6):787-93. doi: 10.1016/j.metabol.2011.10.011. Epub 2011 Dec 28. Metabolism. 2012. PMID: 22209671
-
Ziprasidone in the treatment of affective disorders: a review.CNS Neurosci Ther. 2008 Winter;14(4):278-86. doi: 10.1111/j.1755-5949.2008.00056.x. CNS Neurosci Ther. 2008. PMID: 19040553 Free PMC article. Review.
Cited by
-
Protective effects of novel organic selenium compounds against oxidative stress in the nematode Caenorhabditis elegans.Toxicol Rep. 2015;2:961-967. doi: 10.1016/j.toxrep.2015.06.010. Toxicol Rep. 2015. PMID: 26726309 Free PMC article.
-
Application of Caenorhabditis elegans in Lipid Metabolism Research.Int J Mol Sci. 2023 Jan 7;24(2):1173. doi: 10.3390/ijms24021173. Int J Mol Sci. 2023. PMID: 36674689 Free PMC article. Review.
-
Drug elucidation: invertebrate genetics sheds new light on the molecular targets of CNS drugs.Front Pharmacol. 2014 Jul 28;5:177. doi: 10.3389/fphar.2014.00177. eCollection 2014. Front Pharmacol. 2014. PMID: 25120487 Free PMC article. Review.
-
Nutritional Supplementation Benefits in under Developmental Disruption and Stress Conditions.ACS Omega. 2025 Jul 17;10(29):31313-31330. doi: 10.1021/acsomega.4c10748. eCollection 2025 Jul 29. ACS Omega. 2025. PMID: 40757322 Free PMC article.
-
Synthesis and Physicochemical Stability of a Copaiba Balsam Oil (Copaifera sp.) Nanoemulsion and Prospecting of Toxicological Effects on the Nematode Caenorhabditis elegans.ACS Omega. 2024 Sep 6;9(37):39100-39118. doi: 10.1021/acsomega.4c05930. eCollection 2024 Sep 17. ACS Omega. 2024. PMID: 39310144 Free PMC article.
References
-
- Miyamoto S, Duncan GE, Marx CE, Lieberman JA (2005) Treatments for schizophrenia: a critical review of pharmacology and mechanisms of action of antipsychotic drugs. Mol Psychiatry 10: 79-104. doi:10.1038/sj.mp.4001556. PubMed: 15289815. - DOI - PubMed
-
- Newcomer JW (2007) Metabolic considerations in the use of antipsychotic medications: a review of recent evidence. J Clin Psychiatry 68: 20-27. doi:10.4088/JCP.0807e20. PubMed: 17286524. - DOI - PubMed
-
- Haupt DW (2006) Differential metabolic effects of antipsychotic treatments. Eur Neuropsychopharmacol 16: 149-152. doi:10.1016/j.euroneuro.2006.06.003. PubMed: 16872808. - DOI - PubMed
-
- Wang PW, Hill SJ, Childers ME, Chandler RA, Rasgon NL et al. (2011) Open adjunctive ziprasidone associated with weight loss in obese and overweight bipolar disorder patients. J Psychiatr Res 45: 1128-1132. doi:10.1016/j.jpsychires.2011.01.019. PubMed: 21371718. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

