Mosquito-parasite interactions can shape filariasis transmission dynamics and impact elimination programs
- PMID: 24069488
- PMCID: PMC3772046
- DOI: 10.1371/journal.pntd.0002433
Mosquito-parasite interactions can shape filariasis transmission dynamics and impact elimination programs
Abstract
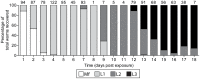
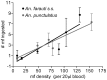
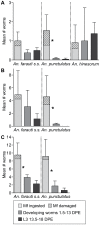
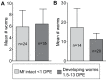
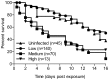
The relationship between mosquito vectors and lymphatic filariasis (LF) parasites can result in a range of transmission outcomes. Anophelines are generally characterized as poor vectors due to an inability to support development at low densities. However, it is important to understand the potential for transmission in natural vectors to maximize the success of elimination efforts. Primary vectors in Papua New Guinea (n = 1209) were dissected following exposure to microfilaremic blood (range 8-233 mf/20 µl). We examined density dependent and species-specific parasite prevalence, intensity and yield, barriers to parasite development as well as impacts on mosquito survival. We observed strikingly different parasite prevalence and yield among closely related species. Prevalence of infective stage larvae (L3s) ranged from 4.2% to 23.7% in An. punctulatus, 24.5% to 68.6% in An. farauti s.s. and 61.9% to 100% in An. hinesorum at low and high density exposures, respectively. Injection experiments revealed the greatest barrier to parasite development involved passage from the midgut into the hemocoel. The ratio of L3 to ingested mf at low densities was higher in An. hinesorum (yield = 1.0) and An. farauti s.s. (yield = 0.5) than has been reported in other anopheline vectors. There was a negative relationship between mosquito survival and bloodmeal mf density. In An. farauti s.s., increased parasite yield and survival at low densities suggest greater competence at low microfilaremias. In Papua New Guinea the likelihood of transmission will be strongly influenced by vector composition and changes in the mf reservoir as a result of elimination efforts. Global elimination efforts will be strengthened by the knowledge of transmission potential in the context of current control measures.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- WHO (2008) The global burden of disease: 2004 update. Geneva: WHO.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

