The role of changes in extracellular matrix of cartilage in the presence of inflammation on the pathology of osteoarthritis
- PMID: 24069595
- PMCID: PMC3771246
- DOI: 10.1155/2013/284873
The role of changes in extracellular matrix of cartilage in the presence of inflammation on the pathology of osteoarthritis
Abstract
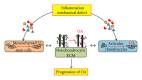
Osteoarthritis (OA) is a degenerative disease that affects various tissues surrounding joints such as articular cartilage, subchondral bone, synovial membrane, and ligaments. No therapy is currently available to completely prevent the initiation or progression of the disease partly due to poor understanding of the mechanisms of the disease pathology. Cartilage is the main tissue afflicted by OA, and chondrocytes, the sole cellular component in the tissue, actively participate in the degeneration process. Multiple factors affect the development and progression of OA including inflammation that is sustained during the progression of the disease and alteration in biomechanical conditions due to wear and tear or trauma in cartilage. During the progression of OA, extracellular matrix (ECM) of cartilage is actively remodeled by chondrocytes under inflammatory conditions. This alteration of ECM, in turn, changes the biomechanical environment of chondrocytes, which further drives the progression of the disease in the presence of inflammation. The changes in ECM composition and structure also prevent participation of mesenchymal stem cells in the repair process by inhibiting their chondrogenic differentiation. This review focuses on how inflammation-induced ECM remodeling disturbs cellular activities to prevent self-regeneration of cartilage in the pathology of OA.
Figures

References
-
- Pearle AD, Warren RF, Rodeo SA. Basic science of articular cartilage and osteoarthritis. Clinics in Sports Medicine. 2005;24(1):1–12. - PubMed
-
- Martel-Pelletier J, Boileau C, Pelletier J, Roughley PJ. Cartilage in normal and osteoarthritis conditions. Best Practice and Research. 2008;22(2):351–384. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

