Activation of the ERK1/2 signaling pathway during the osteogenic differentiation of mesenchymal stem cells cultured on substrates modified with various chemical groups
- PMID: 24069599
- PMCID: PMC3771309
- DOI: 10.1155/2013/361906
Activation of the ERK1/2 signaling pathway during the osteogenic differentiation of mesenchymal stem cells cultured on substrates modified with various chemical groups
Abstract
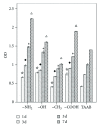
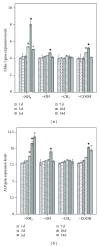
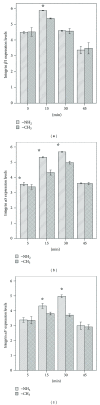
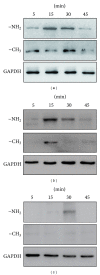
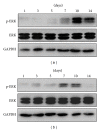
The current study examined the influence of culture substrates modified with the functional groups -OH, -COOH, -NH2, and -CH3 using SAMs technology, in conjunction with TAAB control, on the osteogenic differentiation of rabbit BMSCs. The CCK-8 assay revealed that BMSCs exhibited substrate-dependent cell viability. The cells plated on -NH2- and -OH-modified substrates were well spread and homogeneous, but those on the -COOH- and -CH3-modified substrates showed more rounded phenotype. The mRNA expression of BMSCs revealed that -NH2-modified substrate promoted the mRNA expression and osteogenic differentiation of the BMSCs. The contribution of ERK1/2 signaling pathway to the osteogenic differentiation of BMSCs cultured on the -NH2-modified substrate was investigated in vitro. The -NH2-modified substrate promoted the expression of integrins; the activation of FAK and ERK1/2. Inhibition of ERK1/2 activation by PD98059, a specific inhibitor of the ERK signaling pathway, blocked ERK1/2 activation in a dose-dependent manner, as revealed for expression of Cbf α -1 and ALP. Blockade of ERK1/2 phosphorylation in BMSCs by PD98059 suppressed osteogenic differentiation on chemical surfaces. These findings indicate a potential role for ERK in the osteogenic differentiation of BMSCs on surfaces modified by specific chemical functional groups, indicating that the microenvironment affects the differentiation of BMSCs. This observation has important implications for bone tissue engineering.
Figures





References
-
- Buma P, Schreurs W, Verdonschot N. Skeletal tissue engineering—from in vitro studies to large animal models. Biomaterials. 2004;25(9):1487–1495. - PubMed
-
- Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchy-mal stem cells. Science. 1999;284(5411):143–147. - PubMed
-
- Dallari D, Fini M, Stagni C, et al. In vivo study on the healing of bone defects treated with bone marrow stromal cells, platelet-rich plasma, and freeze-dried bone allografts, alone and in combination. Journal of Orthopaedic Research. 2006;24(5):877–888. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

