Tissue sparing, behavioral recovery, supraspinal axonal sparing/regeneration following sub-acute glial transplantation in a model of spinal cord contusion
- PMID: 24070030
- PMCID: PMC3849889
- DOI: 10.1186/1471-2202-14-106
Tissue sparing, behavioral recovery, supraspinal axonal sparing/regeneration following sub-acute glial transplantation in a model of spinal cord contusion
Abstract
Background: It has been shown that olfactory ensheathing glia (OEG) and Schwann cell (SCs) transplantation are beneficial as cellular treatments for spinal cord injury (SCI), especially acute and sub-acute time points. In this study, we transplanted DsRED transduced adult OEG and SCs sub-acutely (14 days) following a T10 moderate spinal cord contusion injury in the rat. Behaviour was measured by open field (BBB) and horizontal ladder walking tests to ascertain improvements in locomotor function. Fluorogold staining was injected into the distal spinal cord to determine the extent of supraspinal and propriospinal axonal sparing/regeneration at 4 months post injection time point. The purpose of this study was to investigate if OEG and SCs cells injected sub acutely (14 days after injury) could: (i) improve behavioral outcomes, (ii) induce sparing/regeneration of propriospinal and supraspinal projections, and (iii) reduce tissue loss.
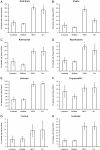
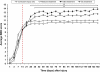
Results: OEG and SCs transplanted rats showed significant increased locomotion when compared to control injury only in the open field tests (BBB). However, the ladder walk test did not show statistically significant differences between treatment and control groups. Fluorogold retrograde tracing showed a statistically significant increase in the number of supraspinal nuclei projecting into the distal spinal cord in both OEG and SCs transplanted rats. These included the raphe, reticular and vestibular systems. Further pairwise multiple comparison tests also showed a statistically significant increase in raphe projecting neurons in OEG transplanted rats when compared to SCs transplanted animals. Immunohistochemistry of spinal cord sections short term (2 weeks) and long term (4 months) showed differences in host glial activity, migration and proteoglycan deposits between the two cell types. Histochemical staining revealed that the volume of tissue remaining at the lesion site had increased in all OEG and SCs treated groups. Significant tissue sparing was observed at both time points following glial SCs transplantation. In addition, OEG transplants showed significantly decreased chondroitin proteoglycan synthesis in the lesion site, suggesting a more CNS tolerant graft.
Conclusions: These results show that transplantation of OEG and SCs in a sub-acute phase can improve anatomical outcomes after a contusion injury to the spinal cord, by increasing the number of spared/regenerated supraspinal fibers, reducing cavitation and enhancing tissue integrity. This provides important information on the time window of glial transplantation for the repair of the spinal cord.
Figures








References
-
- Ruitenberg MJ, Vukovic J, Sarich J, Busfield SJ, Plant GW. Olfactory ensheathing cells: characteristics genetic engineering and therapeutic potential. J Neurotrauma. 2006;23(3–4):468–478. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

