Bone regeneration potential of stem cells derived from periodontal ligament or gingival tissue sources encapsulated in RGD-modified alginate scaffold
- PMID: 24070211
- PMCID: PMC3926152
- DOI: 10.1089/ten.TEA.2013.0229
Bone regeneration potential of stem cells derived from periodontal ligament or gingival tissue sources encapsulated in RGD-modified alginate scaffold
Abstract
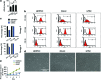
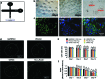
Mesenchymal stem cells (MSCs) provide an advantageous alternative therapeutic option for bone regeneration in comparison to current treatment modalities. However, delivering MSCs to the defect site while maintaining a high MSC survival rate is still a critical challenge in MSC-mediated bone regeneration. Here, we tested the bone regeneration capacity of periodontal ligament stem cells (PDLSCs) and gingival mesenchymal stem cells (GMSCs) encapsulated in a novel RGD- (arginine-glycine-aspartic acid tripeptide) coupled alginate microencapsulation system in vitro and in vivo. Five-millimeter-diameter critical-size calvarial defects were created in immunocompromised mice and PDLSCs and GMSCs encapsulated in RGD-modified alginate microspheres were transplanted into the defect sites. New bone formation was assessed using microcomputed tomography and histological analyses 8 weeks after transplantation. Results confirmed that our microencapsulation system significantly enhanced MSC viability and osteogenic differentiation in vitro compared with non-RGD-containing alginate hydrogel microspheres with larger diameters. Results confirmed that PDLSCs were able to repair the calvarial defects by promoting the formation of mineralized tissue, while GMSCs showed significantly lower osteogenic differentiation capability. Further, results revealed that RGD-coupled alginate scaffold facilitated the differentiation of oral MSCs toward an osteoblast lineage in vitro and in vivo, as assessed by expression of osteogenic markers Runx2, ALP, and osteocalcin. In conclusion, these results for the first time demonstrated that MSCs derived from orofacial tissue encapsulated in RGD-modified alginate scaffold show promise for craniofacial bone regeneration. This treatment modality has many potential dental and orthopedic applications.
Figures



References
-
- Sachlos E., and Czernuszka J.T.Making tissue engineering scaffolds work. Review on the application of solid freeform fabrication technology to the production of tissue engineering scaffold. Eur Cell Mater 5,29, 2003 - PubMed
-
- Chen F.M., Zhang J., Zhang M., An Y., Chen F., and Wu Z.F.A review on endogenous regenerative technology in periodontal regenerative medicine. Biomaterials 31,7892, 2010 - PubMed
-
- Monaco E., Bionaz M., Hollister S.J., and Wheeler M.B.Strategies for regeneration of the bone using porcine adult adipose-derived mesenchymal stem cells. Theriogenology 75,1381, 2011 - PubMed
-
- Seo B.M., Miura M., Gronthos S., Bartold P.M., Batouli S., Brahim J., et al. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 364,149, 2004 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

