Neuroprotective efficacy of a proneurogenic compound after traumatic brain injury
- PMID: 24070637
- PMCID: PMC3934600
- DOI: 10.1089/neu.2013.3135
Neuroprotective efficacy of a proneurogenic compound after traumatic brain injury
Abstract
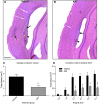
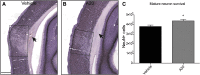
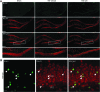
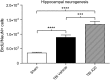
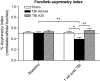
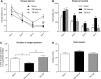
Traumatic brain injury (TBI) is characterized by histopathological damage and long-term sensorimotor and cognitive dysfunction. Recent studies have reported the discovery of the P7C3 class of aminopropyl carbazole agents with potent neuroprotective properties for both newborn neural precursor cells in the adult hippocampus and mature neurons in other regions of the central nervous system. This study tested, for the first time, whether the highly active P7C3-A20 compound would be neuroprotective, promote hippocampal neurogenesis, and improve functional outcomes after experimental TBI. Sprague-Dawley rats subjected to moderate fluid percussion brain injury were evaluated for quantitative immunohistochemical and behavioral changes after trauma. P7C3-A20 (10 mg/kg) or vehicle was initiated intraperitoneally 30 min postsurgery and twice per day every day thereafter for 7 days. Administration of P7C3-A20 significantly reduced overall contusion volume, preserved vulnerable anti-neuronal nuclei (NeuN)-positive pericontusional cortical neurons, and improved sensorimotor function 1 week after trauma. P7C3-A20 treatment also significantly increased both bromodeoxyuridine (BrdU)- and doublecortin (DCX)-positive cells within the subgranular zone of the ipsilateral dentate gyrus 1 week after TBI. Five weeks after TBI, animals treated with P7C3-A20 showed significantly increased BrdU/NeuN double-labeled neurons and improved cognitive function in the Morris water maze, compared to TBI-control animals. These results suggest that P7C3-A20 is neuroprotective and promotes endogenous reparative strategies after TBI. We propose that the chemical scaffold represented by P7C3-A20 provides a basis for optimizing and advancing new pharmacological agents for protecting patients against the early and chronic consequences of TBI.
Figures

References
-
- Langlois J.A., and Rutland-Brown W. (2005) Traumatic Brain Injury in the United States: The Future of Registries and Data Systems. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control: Atlanta, GA
-
- Bramlett H.M., and Dietrich W.D. (2007). Progressive damage after brain and spinal cord injury: pathomechanisms and treatment strategies. Prog. Brain Res. 161, 125–141 - PubMed
-
- Kotapka M.J., Gennarelli T.A., Graham D.I., Adams J.H., Thibault L.E., Ross D.T., and Ford I. (1991). Selective vulnerability of hippocampal neurons in acceleration-induced experimental head injury. J. Neurotrauma 8, 247–258 - PubMed
-
- Bramlett H.M., Green E.J., and Dietrich W.D. (1997). Hippocampally dependent and independent chronic spatial navigational deficits following parasagittal fluid percussion brain injury in the rat. Brain Res. 762, 195–202 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

