Constitutive stabilization of hypoxia-inducible factor alpha selectively promotes the self-renewal of mesenchymal progenitors and maintains mesenchymal stromal cells in an undifferentiated state
- PMID: 24071737
- PMCID: PMC3789268
- DOI: 10.1038/emm.2013.87
Constitutive stabilization of hypoxia-inducible factor alpha selectively promotes the self-renewal of mesenchymal progenitors and maintains mesenchymal stromal cells in an undifferentiated state
Abstract
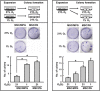
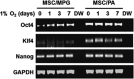
With the increasing use of culture-expanded mesenchymal stromal cells (MSCs) for cell therapies, factors that regulate the cellular characteristics of MSCs have been of major interest. Oxygen concentration has been shown to influence the functions of MSCs, as well as other normal and malignant stem cells. However, the underlying mechanisms of hypoxic responses and the precise role of hypoxia-inducible factor-1α (Hif-1α), the master regulatory protein of hypoxia, in MSCs remain unclear, due to the limited span of Hif-1α stabilization and the complex network of hypoxic responses. In this study, to further define the significance of Hif-1α in MSC function during their self-renewal and terminal differentiation, we established adult bone marrow (BM)-derived MSCs that are able to sustain high level expression of ubiquitin-resistant Hif-1α during such long-term biological processes. Using this model, we show that the stabilization of Hif-1α proteins exerts a selective influence on colony-forming mesenchymal progenitors promoting their self-renewal and proliferation, without affecting the proliferation of the MSC mass population. Moreover, Hif-1α stabilization in MSCs led to the induction of pluripotent genes (oct-4 and klf-4) and the inhibition of their terminal differentiation into osteogenic and adipogenic lineages. These results provide insights into the previously unrecognized roles of Hif-1α proteins in maintaining the primitive state of primary MSCs and on the cellular heterogeneities in hypoxic responses among MSC populations.
Figures





References
-
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. - PubMed
-
- Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74. - PubMed
-
- Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076–1084. - PubMed
-
- Giordano A, Galderisi U, Marino IR. From the laboratory bench to the patient's bedside: an update on clinical trials with mesenchymal stem cells. J Cell Physiol. 2007;211:27–35. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
