Autologous transplantation of GDNF-expressing mesenchymal stem cells protects against MPTP-induced damage in cynomolgus monkeys
- PMID: 24071770
- PMCID: PMC4070584
- DOI: 10.1038/srep02786
Autologous transplantation of GDNF-expressing mesenchymal stem cells protects against MPTP-induced damage in cynomolgus monkeys
Abstract
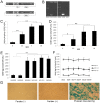
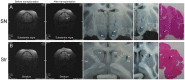
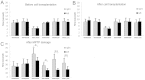
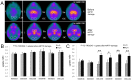
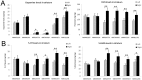
Glial cell-derived neurotrophic factor (GDNF) has shown beneficial effects in models of Parkinson's disease. The mild results observed in the double-blind clinical trial by intraputamenal infusion of recombinant GDNF proteins warrant a search for alternative delivery methods. In this study, we investigated the function of autologous mesenchymal stem cells (MSCs) expressing GDNF (GDNF-MSCs) for protection against MPTP-induced injury in cynomolgus monkeys. MSCs were obtained from the bone marrow of individual monkeys and gene-modified to express GDNF. Following unilateral engraftment of GDNF-MSCs into the striatum and substantia nigra, the animals were challenged with MPTP to induce a stable systemic Parkinsonian state. The motor functions were spared in the contralateral limbs of monkeys receiving GDNF-MSCs, but not in those receiving MSCs alone. In the striatum of the grafted hemisphere, dopamine levels were higher and dopamine uptake was enhanced. The results suggest that autologous MSCs may be a safe vehicle to deliver GDNF for enhancing nigro-striatum functions.
Figures


References
-
- de Rijk M. C. et al. Prevalence of Parkinson's disease in Europe: A collaborative study of population-based cohorts. Neurologic Diseases in the Elderly Research Group. Neurology 54, S21–23 (2000). - PubMed
-
- Choi-Lundberg D. L. et al. Dopaminergic neurons protected from degeneration by GDNF gene therapy. Science 275, 838–841 (1997). - PubMed
-
- Gash D. M. et al. Functional recovery in parkinsonian monkeys treated with GDNF. Nature 380, 252–255 (1996). - PubMed
-
- Tomac A. et al. Protection and repair of the nigrostriatal dopaminergic system by GDNF in vivo. Nature 373, 335–339 (1995). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

