The use of ROC analysis for the qualitative prediction of human oral bioavailability from animal data
- PMID: 24072264
- PMCID: PMC4250569
- DOI: 10.1007/s11095-013-1193-2
The use of ROC analysis for the qualitative prediction of human oral bioavailability from animal data
Abstract
Purpose: To develop and evaluate a tool for the qualitative prediction of human oral bioavailability (Fhuman) from animal oral bioavailability (Fanimal) data employing ROC analysis and to identify the optimal thresholds for such predictions.
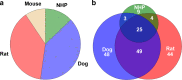
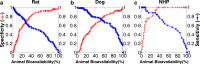
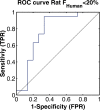
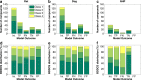
Methods: A dataset of 184 compounds with known Fhuman and Fanimal in at least one species (mouse, rat, dog and non-human primates (NHP)) was employed. A binary classification model for Fhuman was built by setting a threshold for high/low Fhuman at 50%. The thresholds for high/low Fanimal were varied from 0 to 100 to generate the ROC curves. Optimal thresholds were derived from 'cost analysis' and the outcomes with respect to false negative and false positive predictions were analyzed against the BDDCS class distributions.
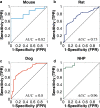
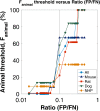
Results: We successfully built ROC curves for the combined dataset and per individual species. Optimal Fanimal thresholds were found to be 67% (mouse), 22% (rat), 58% (dog), 35% (NHP) and 47% (combined dataset). No significant trends were observed when sub-categorizing the outcomes by the BDDCS.
Conclusions: Fanimal can predict high/low Fhuman with adequate sensitivity and specificity. This methodology and associated thresholds can be employed as part of decisions related to planning necessary studies during development of new drug candidates and lead selection.
Figures

References
-
- Lin JH. Species similarities and differences in pharmacokinetics. Drug Metab Dispos. 1995;23:1008–21. - PubMed
-
- Sietsema WK. The absolute oral bioavailability of selected drugs. Int J Clin Pharmacol Ther Toxicol. 1989;27:179–211. - PubMed
-
- Chhabra RS, Pohl RJ, Fouts JR. A comparative study of xenobiotic-metabolizing enzymes in liver and intestine of various animal species. Drug Metab Dispos. 1974;2:443–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

