Loss of NHE3 alters gut microbiota composition and influences Bacteroides thetaiotaomicron growth
- PMID: 24072680
- PMCID: PMC3840232
- DOI: 10.1152/ajpgi.00184.2013
Loss of NHE3 alters gut microbiota composition and influences Bacteroides thetaiotaomicron growth
Abstract
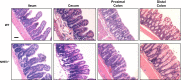
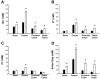
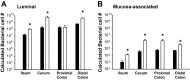
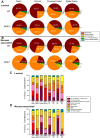
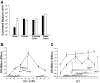
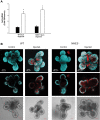
Changes in the intestinal microbiota have been linked to diabetes, obesity, inflammatory bowel disease, and Clostridium difficile (C. difficile)-associated disease. Despite this, it remains unclear how the intestinal environment, set by ion transport, affects luminal and mucosa-associated bacterial composition. Na(+)/H(+)-exchanger isoform 3 (NHE3), a target of C. difficile toxin B, plays an integral role in intestinal Na(+) absorption. Thus the NHE3-deficient mouse model was chosen to examine the effect of pH and ion composition on bacterial growth. We hypothesized that ion transport-induced change in the intestinal environment would lead to alteration of the microbiota. Region-specific changes in ion composition and pH correlated with region-specific alteration of luminal and mucosal-associated bacteria with general decreases in Firmicutes and increases in Bacteroidetes members. Bacteroides thetaiotaomicron (B. thetaiotaomicron) increased in NHE3(-/-) terminal ileum and was examined in vitro to determine whether altered Na(+) was sufficient to affect growth. Increased in vitro growth of B. thetaiotaomicron occurred in 43 mM Na(+) correlating with the NHE3(-/-) mouse terminal ileum [Na(+)]. NHE3(-/-) terminal ileum displayed increased fut2 mRNA and fucosylation correlating with B. thetaiotaomicron growth. Inoculation of B. thetaiotaomicron in wild-type and NHE3(-/-) terminal ileum organoids displayed increased fut2 and fucosylation, indicating that B. thetaiotaomicron alone is sufficient for the increased fucosylation seen in vivo. These data demonstrate that loss of NHE3 alters the intestinal environment, leading to region-specific changes in bacteria, and shed light on the growth requirements of some gut microbiota members, which is vital for creating better treatments of complex diseases with an altered gut microbiota.
Keywords: Bacteroides thetaiotaomicron; Clostridium difficile; Na+/H+-exchanger isoform 3; fucosylation; ileum.
Figures

References
-
- Avetisyan AV, Bogachev AV, Murtasina RA, Skulachev VP. ATP-driven Na+ transport and Na(+)-dependent ATP synthesis in Escherichia coli grown at low delta mu H+. FEBS Lett 317: 267–270, 1993 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

