The inhibitory circuit architecture of the lateral hypothalamus orchestrates feeding
- PMID: 24072922
- PMCID: PMC4131546
- DOI: 10.1126/science.1241812
The inhibitory circuit architecture of the lateral hypothalamus orchestrates feeding
Abstract
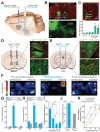
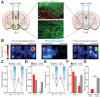
The growing prevalence of overeating disorders is a key contributor to the worldwide obesity epidemic. Dysfunction of particular neural circuits may trigger deviations from adaptive feeding behaviors. The lateral hypothalamus (LH) is a crucial neural substrate for motivated behavior, including feeding, but the precise functional neurocircuitry that controls LH neuronal activity to engage feeding has not been defined. We observed that inhibitory synaptic inputs from the extended amygdala preferentially innervate and suppress the activity of LH glutamatergic neurons to control food intake. These findings help explain how dysregulated activity at a number of unique nodes can result in a cascading failure within a defined brain network to produce maladaptive feeding.
Figures


Comment in
-
Obesity: The need to eat--overruling the homeostatic control of feeding.Nat Rev Endocrinol. 2014 Jan;10(1):5-6. doi: 10.1038/nrendo.2013.235. Epub 2013 Nov 26. Nat Rev Endocrinol. 2014. PMID: 24275739 No abstract available.
References
-
- Hoebel BG, Teitelbaum P. Hypothalamic Control of Feeding and Self-Stimulation. Science. 1962;135:375–377. - PubMed
-
- Delgado JR, Anand BK. Increase of food intake induced by electrical stimulation of the lateral hypothalamus. Am. J. Physiol. 1953;172:162–168. - PubMed
-
- Wise RA. Hypothalamic motivational systems: fixed or plastic neural circuits? Science. 1968;162:377–379. - PubMed
-
- Stanley BG, Ha LH, Spears LC, Dee MG., 2nd Lateral hypothalamic injections of glutamate, kainic acid, D,L-alpha-amino-3-hydroxy-5-methyl isoxazole propionic acid or N-methyl-D-aspartic acid rapidly elicit intense transient eating in rats. Brain Res. 1993;613:88–95. - PubMed
-
- Turenius CI, et al. GABA(A) receptors in the lateral hypothalamus as mediators of satiety and body weight regulation. Brain Res. 2009;1262:16–24. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

