Increased neurofilament light chain blood levels in neurodegenerative neurological diseases
- PMID: 24073237
- PMCID: PMC3779219
- DOI: 10.1371/journal.pone.0075091
Increased neurofilament light chain blood levels in neurodegenerative neurological diseases
Abstract
Objective: Neuronal damage is the morphological substrate of persisting neurological disability. Neurofilaments (Nf) are cytoskeletal proteins of neurons and their release into cerebrospinal fluid has shown encouraging results as a biomarker for neurodegeneration. This study aimed to validate the quantification of the Nf light chain (NfL) in blood samples, as a biofluid source easily accessible for longitudinal studies.
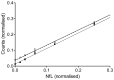
Methods: We developed and applied a highly sensitive electrochemiluminescence (ECL) based immunoassay for quantification of NfL in blood and CSF.
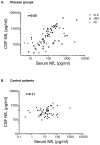
Results: Patients with Alzheimer's disease (AD) (30.8 pg/ml, n=20), Guillain-Barré-syndrome (GBS) (79.4 pg/ml, n=19) or amyotrophic lateral sclerosis (ALS) (95.4 pg/ml, n=46) had higher serum NfL values than a control group of neurological patients without evidence of structural CNS damage (control patients, CP) (4.4 pg/ml, n=68, p<0.0001 for each comparison, p=0.002 for AD patients) and healthy controls (HC) (3.3 pg/ml, n=67, p<0.0001). Similar differences were seen in corresponding CSF samples. CSF and serum levels correlated in AD (r=0.48, p=0.033), GBS (r=0.79, p<0.0001) and ALS (r=0.70, p<0.0001), but not in CP (r=0.11, p=0.3739). The sensitivity and specificity of serum NfL for separating ALS from healthy controls was 91.3% and 91.0%.
Conclusions: We developed and validated a novel ECL based sandwich immunoassay for the NfL protein in serum (NfL(Umea47:3)); levels in ALS were more than 20-fold higher than in controls. Our data supports further longitudinal studies of serum NfL in neurodegenerative diseases as a potential biomarker of on-going disease progression, and as a potential surrogate to quantify effects of neuroprotective drugs in clinical trials.
Conflict of interest statement
Figures



References
-
- Petzold A (2005) Neurofilament phosphoforms: surrogate markers for axonal injury, degeneration and loss. J Neurol Sci 233: 183-198. doi:10.1016/j.jns.2005.03.015. PubMed: 15896809. - DOI - PubMed
-
- Brettschneider J, Petzold A, Junker A, Tumani H (2006) Axonal damage markers in the cerebrospinal fluid of patients with clinically isolated syndrome improve predicting conversion to definite multiple sclerosis. Mult Scler 12: 143-148. doi:10.1191/135248506ms1263oa. PubMed: 16629417. - DOI - PubMed
-
- Deisenhammer F, Egg R, Giovannoni G, Hemmer B, Petzold A et al. (2009) EFNS guidelines on disease-specific CSF investigations. Eur J Neurol 16: 760-770. doi:10.1111/j.1468-1331.2009.02595.x. PubMed: 19475759. - DOI - PubMed
-
- Giovannoni G (2010) Cerebrospinal fluid neurofilament: the biomarker that will resuscitate the 'Spinal Tap'. Mult Scler 16: 285-286. 16/3/285 [PII]; doi:10.1177/1352458510361358 - DOI - PubMed
-
- Gunnarsson M, Malmeström C, Axelsson M, Sundström P, Dahle C et al. (2011) Axonal damage in relapsing multiple sclerosis is markedly reduced by natalizumab. Ann Neurol 69: 83-89. doi:10.1002/ana.22247. PubMed: 21280078. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

