The duration of Chlamydia muridarum genital tract infection and associated chronic pathological changes are reduced in IL-17 knockout mice but protection is not increased further by immunization
- PMID: 24073293
- PMCID: PMC3779189
- DOI: 10.1371/journal.pone.0076664
The duration of Chlamydia muridarum genital tract infection and associated chronic pathological changes are reduced in IL-17 knockout mice but protection is not increased further by immunization
Abstract
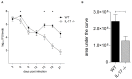
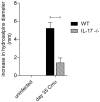
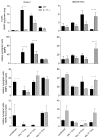
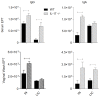
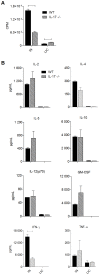
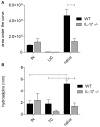
IL-17 is believed to be important for protection against extracellular pathogens, where clearance is dependent on neutrophil recruitment and local activation of epithelial cell defences. However, the role of IL-17 in protection against intracellular pathogens such as Chlamydia is less clear. We have compared (i) the course of natural genital tract C. muridarum infection, (ii) the development of oviduct pathology and (iii) the development of vaccine-induced immunity against infection in wild type (WT) BALB/c and IL-17 knockout mice (IL-17-/-) to determine if IL-17-mediated immunity is implicated in the development of infection-induced pathology and/or protection. Both the magnitude and duration of genital infection was significantly reduced in IL-17-/- mice compared to BALB/c. Similarly, hydrosalpinx was also greatly reduced in IL-17-/- mice and this correlated with reduced neutrophil and macrophage infiltration of oviduct tissues. Matrix metalloproteinase (MMP) 9 and MMP2 were increased in WT oviducts compared to IL-17-/- animals at day 7 post-infection. In contrast, oviducts from IL-17-/- mice contained higher MMP9 and MMP2 at day 21. Infection also elicited higher levels of Chlamydia-neutralizing antibody in serum of IL-17-/- mice than WT mice. Following intranasal immunization with C. muridarumMajor Outer Membrane Protein (MOMP) and cholera toxin plus CpG adjuvants, significantly higher levels of chlamydial MOMP-specific IgG and IgA were found in serum and vaginal washes of IL-17-/- mice. T cell proliferation and IFNγ production by splenocytes was greater in WT animals following in vitro re-stimulation, however vaccination was only effective at reducing infection in WT, not IL-17-/- mice. Intranasal or transcutaneous immunization protected WT but not IL-17-/- mice against hydrosalpinx development. Our data show that in the absence of IL-17, the severity of C. muridarum genital infection and associated oviduct pathology are significantly attenuated, however neither infection or pathology can be reduced further by vaccination protocols that effectively protect WT mice.
Conflict of interest statement
Figures


References
-
- Beagley KW, Timms P (2000) Chlamydia trachomatis infection: incidence, health costs and prospects for vaccine development. J Reprod Immunol 48: 47-68. doi:10.1016/S0165-0378(00)00069-3. PubMed: 10996382. - DOI - PubMed
-
- Morrison RP, Caldwell HD (2002) Immunity to murine chlamydial genital infection. Infect Immun 70: 2741-2751. doi:10.1128/IAI.70.6.2741-2751.2002. PubMed: 12010958. - DOI - PMC - PubMed
-
- Cochrane M, Armitage CW, O’Meara CP, Beagley KW (2010) Towards a Chlamydia trachomatis vaccine: how close are we? Future Microbiol 5: 1833-1856. doi:10.2217/fmb.10.148. PubMed: 21155665. - DOI - PubMed
-
- Fling SP, Sutherland RA, Steele LN, Hess B, D’Orazio SE et al. (2001) CD8+ T cells recognize an inclusion membrane-associated protein from the vacuolar pathogen Chlamydia trachomatis. Proc Natl Acad Sci U S A 98: 1160-1165. doi:10.1073/pnas.98.3.1160. PubMed: 11158611. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

