Long-acting methylphenidate formulations in the treatment of attention-deficit/hyperactivity disorder: a systematic review of head-to-head studies
- PMID: 24074240
- PMCID: PMC3852277
- DOI: 10.1186/1471-244X-13-237
Long-acting methylphenidate formulations in the treatment of attention-deficit/hyperactivity disorder: a systematic review of head-to-head studies
Erratum in
-
Erratum to: Long-acting methylphenidate formulations in the treatment of attention-deficit/hyperactivity disorder: a systematic review of head-to-head studies.BMC Psychiatry. 2015 Aug 25;15:202. doi: 10.1186/s12888-015-0581-z. BMC Psychiatry. 2015. PMID: 26302778 Free PMC article. No abstract available.
Abstract
Background: The stimulant methylphenidate (MPH) has been a mainstay of treatment for attention-deficit/hyperactivity disorder (ADHD) for many years. Owing to the short half-life and the issues associated with multiple daily dosing of immediate-release MPH formulations, a new generation of long-acting MPH formulations has emerged. Direct head-to-head studies of these long-acting MPH formulations are important to facilitate an evaluation of their comparative pharmacokinetics and efficacy; however, to date, relatively few head-to-head studies have been performed.The objective of this systematic review was to compare the evidence available from head-to-head studies of long-acting MPH formulations and provide information that can guide treatment selection.
Methods: A systematic literature search was conducted in MEDLINE and PsycINFO in March 2012 using the MeSH terms: attention deficit disorder with hyperactivity/drug therapy; methylphenidate/therapeutic use and All Fields: Concerta; Ritalin LA; OROS and ADHD; Medikinet; Equasym XL and ADHD; long-acting methylphenidate; Diffucaps and ADHD; SODAS and methylphenidate. No filters were applied and no language, publication date or publication status limitations were imposed. Articles were selected if the title indicated a comparison of two or more long-acting MPH preparations in human subjects of any age; non-systematic review articles and unpublished data were not included.
Results: Of 15,295 references returned in the literature search and screened by title, 34 articles were identified for inclusion: nine articles from pharmacokinetic studies (nine studies); nine articles from laboratory school studies (six studies); two articles from randomized controlled trials (two studies); three articles from switching studies (two studies) and three articles from one observational study.
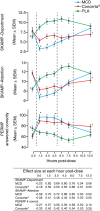
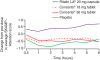
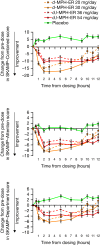
Conclusions: Emerging head-to-head studies provide important data on the comparative efficacy of the formulations available. At a group level, efficacy across the day generally follows the pharmacokinetic profile of the MPH formulation. No formulation is clearly superior to another; careful consideration of patient needs and subtle differences between formulations is required to optimize treatment. For patients achieving suboptimal symptom control, switching long-acting MPH formulations may be beneficial. When switching formulations, it is usually appropriate to titrate the immediate-release component of the formulation; a limitation of current studies is a focus on total daily dose rather than equivalent immediate-release components. Further studies are necessary to provide guidance in clinical practice, particularly in the treatment of adults and pre-school children and the impact of comorbidities and symptom severity on treatment response.
Figures
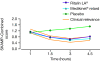
References
-
- Kooij SJ, Bejerot S, Blackwell A, Caci H, Casas-Brugue M, Carpentier PJ, Edvinsson D, Fayyad J, Foeken K, Fitzgerald M, Gaillac V, Ginsberg Y, Henry C, Krause J, Lensing MB, Manor I, Niederhofer H, Nunes-Filipe C, Ohlmeier MD, Oswald P, Pallanti S, Pehlivanidis A, Ramos-Quiroga JA, Rastam M, Ryffel-Rawak D, Stes S, Asherson P. European consensus statement on diagnosis and treatment of adult ADHD: The European Network Adult ADHD. BMC Psychiatry. 2010;10:67. doi: 10.1186/1471-244X-10-67. - DOI - PMC - PubMed
-
- Wolraich M, Brown L, Brown RT, DuPaul G, Earls M, Feldman HM, Ganiats TG, Kaplanek B, Meyer B, Perrin J, Pierce K, Reiff M, Stein MT, Visser S. ADHD: clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics. 2011;128:1007–1022. - PMC - PubMed
-
- Greenhill LL, Pliszka S, Dulcan MK, Bernet W, Arnold V, Beitchman J, Benson RS, Bukstein O, Kinlan J, McClellan J, Rue D, Shaw JA, Stock S. Practice parameter for the use of stimulant medications in the treatment of children, adolescents, and adults. J Am Acad Child Adolesc Psychiatry. 2002;41:26S–49S. - PubMed
-
- Ritalin SR® prescribing information. http://www.pharma.us.novartis.com/product/pi/pdf/ritalin_ritalin-sr.pdf. Date accessed 10/1/13.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

