Mitofusin 2 in POMC neurons connects ER stress with leptin resistance and energy imbalance
- PMID: 24074867
- PMCID: PMC3839088
- DOI: 10.1016/j.cell.2013.09.003
Mitofusin 2 in POMC neurons connects ER stress with leptin resistance and energy imbalance
Abstract
Mitofusin 2 (MFN2) plays critical roles in both mitochondrial fusion and the establishment of mitochondria-endoplasmic reticulum (ER) interactions. Hypothalamic ER stress has emerged as a causative factor for the development of leptin resistance, but the underlying mechanisms are largely unknown. Here, we show that mitochondria-ER contacts in anorexigenic pro-opiomelanocortin (POMC) neurons in the hypothalamus are decreased in diet-induced obesity. POMC-specific ablation of Mfn2 resulted in loss of mitochondria-ER contacts, defective POMC processing, ER stress-induced leptin resistance, hyperphagia, reduced energy expenditure, and obesity. Pharmacological relieve of hypothalamic ER stress reversed these metabolic alterations. Our data establish MFN2 in POMC neurons as an essential regulator of systemic energy balance by fine-tuning the mitochondrial-ER axis homeostasis and function. This previously unrecognized role for MFN2 argues for a crucial involvement in mediating ER stress-induced leptin resistance.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures
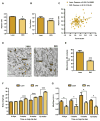

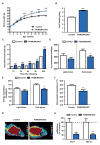
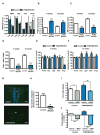
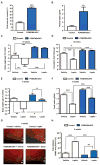
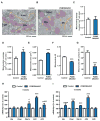
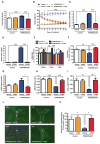
Comment in
-
Mitofusins: mighty regulators of metabolism.Cell. 2013 Sep 26;155(1):17-8. doi: 10.1016/j.cell.2013.09.013. Cell. 2013. PMID: 24074856
-
Neuroendocrinology: Mitofusins and energy balance.Nat Rev Endocrinol. 2013 Dec;9(12):691. doi: 10.1038/nrendo.2013.202. Epub 2013 Oct 15. Nat Rev Endocrinol. 2013. PMID: 24126482 No abstract available.
References
-
- Bach D, Naon D, Pich S, Soriano FX, Vega N, Rieusset J, Laville M, Guillet C, Boirie Y, Wallberg-Henriksson H, et al. Expression of Mfn2, the Charcot-Marie-Tooth neuropathy type 2A gene, in human skeletal muscle: effects of type 2 diabetes, obesity, weight loss, and the regulatory role of tumor necrosis factor alpha and interleukin-6. Diabetes. 2005;54:2685–2693. - PubMed
-
- Bach D, Pich S, Soriano FX, Vega N, Baumgartner B, Oriola J, Daugaard JR, Lloberas J, Camps M, Zierath JR, et al. Mitofusin-2 determines mitochondrial network architecture and mitochondrial metabolism. A novel regulatory mechanism altered in obesity. J Biol Chem. 2003;278:17190–17197. - PubMed
-
- Baltzer C, Tiefenbock SK, Frei C. Mitochondria in response to nutrients and nutrient-sensitive pathways. Mitochondrion. 2010;10:589–597. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

