Maternal and offspring pools of osteocalcin influence brain development and functions
- PMID: 24074871
- PMCID: PMC3864001
- DOI: 10.1016/j.cell.2013.08.042
Maternal and offspring pools of osteocalcin influence brain development and functions
Abstract
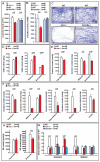
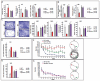
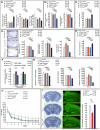
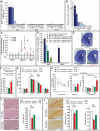
The powerful regulation of bone mass exerted by the brain suggests the existence of bone-derived signals modulating this regulation or other functions of the brain. We show here that the osteoblast-derived hormone osteocalcin crosses the blood-brain barrier, binds to neurons of the brainstem, midbrain, and hippocampus, enhances the synthesis of monoamine neurotransmitters, inhibits GABA synthesis, prevents anxiety and depression, and favors learning and memory independently of its metabolic functions. In addition to these postnatal functions, maternal osteocalcin crosses the placenta during pregnancy and prevents neuronal apoptosis before embryos synthesize this hormone. As a result, the severity of the neuroanatomical defects and learning and memory deficits of Osteocalcin(-/-) mice is determined by the maternal genotype, and delivering osteocalcin to pregnant Osteocalcin(-/-) mothers rescues these abnormalities in their Osteocalcin(-/-) progeny. This study reveals that the skeleton via osteocalcin influences cognition and contributes to the maternal influence on fetal brain development.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures


Comment in
-
Bone: Osteocalcin influences fetal brain development and adult brain function.Nat Rev Endocrinol. 2013 Dec;9(12):689. doi: 10.1038/nrendo.2013.200. Epub 2013 Oct 8. Nat Rev Endocrinol. 2013. PMID: 24100267 No abstract available.
References
-
- Banks WA, Kastin AJ, Huang W, Jaspan JB, Maness LM. Leptin enters the brain by a saturable system independent of insulin. Peptides. 1996;17:305–311. - PubMed
-
- Challis JR. Endocrine disorders in pregnancy: Stress responses in children after maternal glucocorticoids. Nature reviews Endocrinology. 2012;8:629–630. - PubMed
-
- Crawley JN. Exploratory behavior models of anxiety in mice. Neuroscience and biobehavioral reviews. 1985;9:37–44. - PubMed
-
- Cryan JF, Mombereau C, Vassout A. The tail suspension test as a model for assessing antidepressant activity: review of pharmacological and genetic studies in mice. Neuroscience and biobehavioral reviews. 2005;29:571–625. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

