BID preferentially activates BAK while BIM preferentially activates BAX, affecting chemotherapy response
- PMID: 24074954
- PMCID: PMC4164233
- DOI: 10.1016/j.molcel.2013.08.048
BID preferentially activates BAK while BIM preferentially activates BAX, affecting chemotherapy response
Abstract
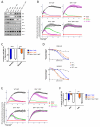
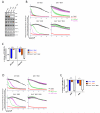
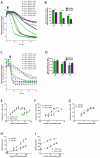
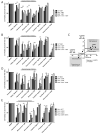
Apoptosis is a highly regulated form of cell death that controls normal homeostasis as well as the antitumor activity of many chemotherapeutic agents. Commitment to death via the mitochondrial apoptotic pathway requires activation of the mitochondrial pore-forming proteins BAK or BAX. Activation can be effected by the activator BH3-only proteins BID or BIM, which have been considered to be functionally redundant in this role. Herein, we show that significant activation preferences exist between these proteins: BID preferentially activates BAK while BIM preferentially activates BAX. Furthermore, we find that cells lacking BAK are relatively resistant to agents that require BID activation for maximal induction of apoptosis, including topoisomerase inhibitors and TRAIL. Consequently, patients with tumors that harbor a loss of BAK1 exhibit an inferior response to topoisomerase inhibitor treatment in the clinic. Therefore, BID and BIM have nonoverlapping roles in the induction of apoptosis via BAK and BAX, affecting chemotherapy response.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures



References
-
- Adachi M, Suematsu S, Kondo T. Targeted mutation in the Fas gene causes hyperplasia in peripheral lymphoid organs and liver. Nature Genetics. 1995 - PubMed
-
- Baldwin EL, Osheroff N. Etoposide, Topoisomerase II and Cancer. Current Medicinal Chemistry - Anti-Cancer Agents. 2005;5:10. - PubMed
-
- Biswas SC, Greene LA. Nerve growth factor (NGF) down-regulates the Bcl-2 homology 3 (BH3) domain-only protein Bim and suppresses its proapoptotic activity by phosphorylation. The Journal of Biological Chemistry. 2002;277:49511–49516. - PubMed
-
- Bouillet P, Metcalf D, Huang DC, Tarlinton DM, Kay TW, Köntgen F, Adams JM, Strasser A. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science (New York, N.Y.) 1999;286:1735–1738. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

