Potential biomarkers of long-term benefit from single-agent trastuzumab or lapatinib in HER2-positive metastatic breast cancer
- PMID: 24075779
- PMCID: PMC5528507
- DOI: 10.1016/j.molonc.2013.08.013
Potential biomarkers of long-term benefit from single-agent trastuzumab or lapatinib in HER2-positive metastatic breast cancer
Abstract
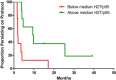
In 2009 a prospective, randomized Phase II trial (NCT00842998) was initiated to evaluate the activity of HER2-targeting agents without chemotherapy (CT) in HER2-positive metastatic breast cancer (MBC) patients. The primary tumors of the patients enrolled in this study offered a unique opportunity to identify biomarkers that could predict durable clinical benefit from CT-free anti-HER2 therapy. Patients with HER2-positive MBC were randomized to trastuzumab or lapatinib as first-line therapy. CT was added to anti-HER2 therapy in patients failing to achieve tumor regression at the 8-week evaluation and in those progressing at any time. Expression analysis of 105 selected genes was performed from formalin-fixed paraffin-embedded primary tumor samples. The research-based PAM50 intrinsic subtypes were also identified. Additionally, quantitative HER2 (H2T) and p95HER2 (p95) protein expression were evaluated by HERmark® and VeraTag® assay, respectively. Predictors of persistence on protocol (PP) were studied by Cox univariate and multivariate analysis. Nineteen patients were enrolled. Median overall survival was 43 months and median PP was 3.8 months (0.8-38.8+), with 4 patients (21.1%) persisting on single agent trastuzumab or lapatinib for longer than 12 mo (14.9-38.8 + mo). Seventeen patients were evaluable for PP. Gene expression analysis revealed that high expression of the 17q12-21 amplicon genes HER2 and GRB7, and the PAM50 HER2-enriched intrinsic profile, were significantly associated with longer PP. Conversely, high expression of luminal-related genes such as PGR, MDM2 or PIK3CA, or the PAM50 luminal intrinsic profile correlated with reduced PP. Moreover, increasing H2T/p95 ratio was found to be significantly associated with longer PP (HR 0.56 per 2-fold increase in H2T/p95, P = 0.0015). Our data suggest that patients belonging to the "HER2-enriched" subtype and/or having high H2T/p95 protein expression ratio are exquisitely sensitive to anti-HER2 agents. MBC patients with these tumors could be candidates for studies aimed at establishing chemotherapy-free regimens.
Keywords: (H2T); (p95); CR; CT; Chemotherapy-free; HER2-enriched; Lapatinib; MR; Microarray; OS; PD; PFS; PP; PR; ROR; SD; Signature; Trastuzumab; chemotherapy; complete remission; minimal response; overall survival; p95HER2; partial remission; persistence on protocol; progression-free survival; progressive disease; risk of relapse; stable disease; total HER2.
Copyright © 2013 Federation of European Biochemical Societies. Published by Elsevier B.V. All rights reserved.
Figures
References
-
- Blackwell, K.L. , Burstein, H.J. , Storniolo, A.M. , 2012. Overall survival benefit with lapatinib in combination with trastuzumab for patients with human epidermal growth factor receptor 2-positive metastatic breast cancer: final results from the EGF104900 Study. J. Clin. Oncol.. 30, 2585–2592. - PubMed
-
- Carey, L.A. , Berry, D. , Ollila, D. , 2013. Clinical and translational results of CALGB 40601: a neoadjuvant phase III trial of weekly paclitaxel and trastuzumab with or without lapatinib for HER2-positive breast cancer. J. Clin. Oncol.. 31, 500 Meeting Abstracts
-
- Cortes, J. , Baselga, J. , Petrella, T. , 2009. Pertuzumab monotherapy following trastuzumab-based treatment: activity and tolerability in patients with advanced HER2- positive breast cancer. J. Clin. Oncol.. 27, 1022 Meeting Abstracts - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous